The International Tinnitus Journal
Official Journal of the Neurootological and Equilibriometric Society
Official Journal of the Brazil Federal District Otorhinolaryngologist Society
ISSN: 0946-5448

Google scholar citation report
Citations : 12717
The International Tinnitus Journal received 12717 citations as per google scholar report
The International Tinnitus Journal peer review process verified at publons
Indexed In
- Excerpta Medica
- Scimago
- SCOPUS
- Publons
- EMBASE
- Google Scholar
- Euro Pub
- CAS Source Index (CASSI)
- Index Medicus
- Medline
- PubMed
- UGC
- EBSCO
Volume 21, Issue 2 / December 2017
Research Paper Pages:157-167
A Randomized, Placebo-Controlled, Double-Blind, Parallel Groups Study Evaluating the Performance and Safety of a Steady State Coherent Biomodulator Patch in the Treatment of Subjective Tinnitus
Authors: Peter Ahnblad, Anders Nordkvist
PDF
Abstract
Objective: The objectives of this study were to evaluate the performance and safety of an innovative passive light photon driven microscopic biomodulator patch as an alternative medical device for tinnitus relief. Materials and Methods: Eighty-two (82) patients were randomized to receive either an active (biomodulator) patch or a placebo patch, for a 3-week treatment period. Patch performance (evaluated with questionnaires related to tinnitus and quality-of-life) and safety were assessed after 3 weeks of treatment (Week 3) and at a follow-up visit 4-weeks after end of treatment (Week 7). Results: The biomodulator patch was safe and well-tolerated and was efficacious, with significant difference (p < 0.05) between the groups at Week 7; active patch had 30% responders compared to 10% for placebo, measured as a decrease from baseline in at least 2 points in tinnitus annoyance visual analogue scale (VAS, 0-10). Tinnitus handicap inventory (THI, 0-100) improved by mean -16 points significantly (p = 0.0005) for the active responder group, but with no statistically significant changes for the placebo group or between the groups. Well-being questionnaire also improved for the active responder group, but not statistically significant. The placebo responder group did not improve in well-being. Other tinnitus related symptoms did not show significant changes. There was no statistically significant difference in performance between the active (biomodulator) and placebo groups directly at the end of treatment (Week 3). Conclusion: In a cost-risk-benefit rationale according to this study it can be reasonable to recommend the biomodulator patch for treatment of tinnitus. Improvements were shown at Week 7 (4 weeks after the end of treatment period).
Keywords: tinnitus, treatment, medical device, patch, coherency, biomodulation.
Introduction
Subjective tinnitus [1] (referred to as tinnitus hereafter) has been defined as a sensory disorder of auditory perception exhibiting an aberrant auditory signal produced by interference in the excitatory-inhibitory process or processes involved in neurotransmission [2] . It seems as if tinnitus is caused by changes in the central nervous system, due to sensory deprivation [3] .
Currently, there is still no US Food and Drug Administration (FDA) or European Medicines Agency (EMA)-approved pharmacological treatment for tinnitus. In the latest clinical practice guidelines for tinnitus (2014), the American Academy of Otolaryngology recommended against the following, for the routine treatment of patients with persistent bothersome tinnitus: (1) antidepressants, anticonvulsants, anxiolytics, or intratympanic medications; (2) Ginkgo biloba, melatonin, zinc, or other dietary supplements and (3) transcranial magnetic stimulation [4] .
Much of today's tinnitus treatment research is focused on neural modulation. For example, oxytocin, a neurohormone and eventual neurotransmitter, has recently studied as a possible tinnitus treatment. In a study comparing intranasal oxytocin to placebo, oxytocin tended to show a reduction in tinnitus distress, although not significantly different to placebo [5] . In addition, intratympanic injections of the synthetic peptide AM-101, presumed to have otoprotective effects, has been studied as a treatment for tinnitus following traumatic cochlear injury. However, AM-101 has yet to demonstrate clear evidence in relief of tinnitus symptoms [6] .
A variety of devices have been used over the years to treat tinnitus but with very limited success. Established neuromodulation therapies for epilepsy and affective disorders, such as vagus nerve stimulation, have been used as novel approaches for tinnitus but without convincing results [7] . The commonly tested and clinically used devices, techniques and therapies for treating tinnitus are: cognitive behavioural therapy (CBT) [8] , which uses relaxation, cognitive restructuring of the thoughts and exposure to exacerbating situations to promote habituation. and is commonly used in moderate to severe cases of tinnitus; tinnitus retraining therapy (TRT) [9] , which is based on a neurophysiological model and consists of a combination of directive counselling and sound therapy in a strict framework and is one of the most commonly used treatment modalities for tinnitus; sound therapy (masking) [10-12] , which is built upon the belief that increasing customized extrinsic sound driven activity of the auditory system results in reduced tinnitus; and repetitive transcranial magnetic stimulation (rTMS) [13] , which is a non-invasive method used to induce electrical currents in the brain, and has received increasing attention in recent years for the treatment of many neuropsychiatric disorders, including tinnitus. Although hearing loss is commonly co-morbid with tinnitus, there is no evidence that prescribing hearing aids routinely relieve tinnitus [14] . Hyperbaric oxygen has been used for idiopathic sudden sensorineural hearing loss with some support of evidence but there is no evidence of a beneficial effect in tinnitus [15] . Acupuncture is used as an alternative method to treat tinnitus, especially in Asia, but most studies are of poor quality and there is no evidence that supports this method today [16] . Low-level laser therapy has so far failed in showing better effect than placebo in treating tinnitus and is routinely not recommended [17] .
Coherency in tinnitus
Resting state recordings for healthy subjects (i.e. not complaining about any tinnitus sound), when in a “to do nothing mode”, are typical with widely distributed networks of coherent brain activation [18, 19] . On the contrary, sufferers of tinnitus are characterized by hyperactivity in the auditory cortex and a changed global brain network [20] . Important increased activation in networks involving the auditory cortex and amygdala have been seen in imaging studies of subjects with tinnitus [21] . In addition, coherence irregularity in quantitative electroencephalography has been identified in a group of patients with severe disabling tinnitus [22] . All the above strongly indicates that coherency is important in maintaining a tinnitus-suppressed equilibrium.
Coherency creating biomodulator
Due to the complexity and heterogeneity of tinnitus, most research regarding possible treatments focus on neuromodulation and neurostimulation. This is because so much depends on the neuronal activity in the brain and the auditory pathways [23, 24] . However, from a broader perspective, it may be important to go from the concept of neuromodulation to the understanding of biomodulation. For example, by including characteristics from the ubiquitous water that are yet to be fully understood. Water accounts for the large majority, 70% mass and 99% of molar concentration of the components of living organisms, i.e. coherent correlations among biomolecules are phase-correlated and should exist in a coherent water state [25] .
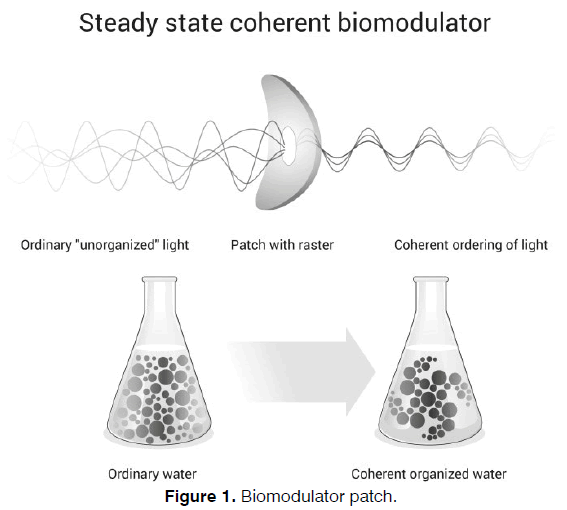
In this study we further explore the effect of an innovative daylight photon driven biomodulator for water coherency on treating tinnitus. In Figure 1 this is shown as a schematic overview image.
This innovative technology was developed in Sweden and has been studied since the 1990s in different research areas. In a previously published open pilot clinical investigation of tinnitus, about half of the patients treated with this biomodulator patch for 21 days experienced relief of symptoms at the end of the 3‑week treatment. Patients continued to experience relief of symptoms one month after the end of the treatment period [26] . In addition, one third of the patients also reported a sustainable relief of their tinnitus up to 2 years after treatment. There were no safety concerns in this pilot study [26] .
Due its unique technology and promising results so far, the possibility of commercialising a nonprescription/ consumer over-the-counter (OTC) device at reasonable expenses it is of great importance to not only better understand and to evaluate this treatment more extensively but also to help many tinnitus sufferers who many not seek health care attention at first.
Materials and Methods
Study design, sample size, monitoring and ethics
The study was ethically approved and independently monitored. The design was a randomized, double-blind, placebo-controlled, parallel-groups clinical investigation. Eighty‑two (82) patients with tinnitus were randomly assigned, in a 1:1 ratio, to a once-daily application of the active (biomodulator) patch or matching placebo patch during a 21-day (3 week) treatment period. Two independent ear-, nose- and throat (ENT) clinics in Sweden recruited patients: one ENT clinic in Gothenburg and one in Stockholm. To ensure that all study related staff were unaware of which patch (active or placebo) the patient received, the investigational products were kept under the responsibility of an independent unblinded person at each site. This unblinded person was not involved in the conduct of the study in any other way than handling of the investigational products, including separate storage of the active (biomodulator) and placebo patches. A single patch was applied, daily, by the patient, behind their left ear in accordance with the “Instructions for Use” provided. The study was conducted in accordance with the principles of the Declaration of Helsinki, the EU guideline MEDDEV 2.7/1 rev. 3, the harmonized standard ISO 141 55 and local regulatory requirements. The overall objective of this study was to evaluate the performance and safety of the active (biomodulator) patch.
The variable used for determining the sample size of this study was the same as the primary variable, i.e. tinnitus annoyance measured on VAS (0-10), where 0 corresponds to “no annoyance” and 10 corresponds to “maximum annoyance”. The assumption was that patients in the active group would get an average decrease (from baseline until day 21) in tinnitus annoyance of 0.5 points more than patients in the placebo group. The standard deviation for the change from baseline until day 21 was assumed to be 0.6 points (based on clinical experience). When the power was set to 90%, the number of fully evaluable patients per treatment group resulted in 31. Including the assumption of 20% drop-out rate, a total of 39 patients per group were to be included, i.e. 78 patients in total needed to be included in the study.
The study consisted of 2 clinic visits (Screening/ randomization at Day 1 and End of treatment at Day 21/ Week 3) and 1 telephone contact (Week 7 [i.e. 4 weeks after end of treatment]). The total duration of the clinical investigation was of 7 weeks, including the screening/ randomization visit (Day 1), the 21-day double-blind treatment period, and the 1-month post-treatment followup period. End-of-treatment evaluations were performed at the end of the double-blind treatment phase (Day 21/ Week 3) or at the time a patient discontinued treatment. The clinical investigation flow chart is shown in Table 1.
| Visit 1 | Visit 2 | Follow up | |
|---|---|---|---|
| Screening, baseline, inclusion | End of treatment | telephone contact 4 weeks after end of treatment | |
| (Week 0) | (Week 3) | (Week 7) | |
| Informed consent | X | ||
| Demography, medical history | X | ||
| Inclusion/exclusion criteria | X | ||
| Physical examination | X | ||
| Dispensing of investigational product | X | ||
| Return of investigational product | X | ||
| Compliance check | X | ||
| Examination of skin area | X | X | |
| VAS tinnitus annoyance | X | X | X |
| VAS hyperacusis rating | X | X | X |
| VAS tinnitus related symptoms | X | X | X |
| Tinnitus Handicap Inventory (THI) | X | X | X |
| Questionnaire on well-being | X | X | X |
| Adverse events/ device effects | X | X | |
| Concomitant medication | X | X | X |
Table 1. Clinical investigation flow chart.
All data was collected in an electronic case report forms (CRFs) and was verified by the Investigator. In addition, a Data Quality Management Plan (DMP) was developed, which provided guidance to ensure that activities met the quality criteria that had been established for complete, consistent and accurate data. The database was locked after completion of data collection and data cleaning.
A 1-year post-study telephone contact was planned to obtain long-term performance and safety results. These results are not included in this publication and will be reported separately later.
Description and suggested mode of action of the patch
The active (biomodulator) patch (Antinitus®, Antinitus AB, Stockholm, Sweden) is an innovation with a patented microscopic fractal and Fourier transforming raster that generates a low frequency, coherent invisible “light” with higher organization, that affects water in general [27] . Water is absolute crucial for human beings and 99 % of the human body molecules comes from water [28, 29] . The raster works passively without any external energy source, Fourier transforming visible and thermal photons that always surround us, even in darkness [27, 30] . The raster turns ordinary water into a coherent and more organized form, creating a spreading self-organization process, which relates on a long-range correlation between water molecules [27] .
The active (biomodulator) patch consists of two layers of polyethylene terephthalate (PET) foil: a bottom perforated foil covered by a glue and a transparent circular foil with an imprint of two concentric silver rings following the proportions of golden section and the Fibonacci series of numbers. The PET foil is imbedded by self-assembling silica clusters forming a natural fractal pattern. The combination of metal clusters with the silica- PET dielectric makes up a nonlinear shift in incident visible and infrared light, which contribute to some novel wave-guiding properties [30] .
The hypothesis is that the active (biomodulator) patch acts as a steady state coherent biomodulator. This is believed to stabilize and desensitize the auditory system, thus resulting in tinnitus relief. It is thought that this is achieved by soothing the chaotic audio-neurosignal loops or what is also known as hyperactivity in a global network of long-range cortical connectivity [20] , which creates the awareness and distress of the internal sound (i.e. tinnitus).
Objectives
The primary objective was:
• To assess the performance of the active (biomodulator) patch relative to placebo on the change of tinnitus symptoms after 21 days (Week 3). The key performance evaluations included tinnitus annoyance visual analogue scale (VAS), which was the primary variable.
The secondary objectives were:
• To assess the performance of the active (biomodulator) patch relative to placebo on the change of tinnitus symptoms 4 weeks after end of treatment (Week 7) compared to baseline. The key performance evaluations included tinnitus annoyance VAS, same as the primary variable.
• To assess if the active (biomodulator) patch could improve the tinnitus patient’s quality of life after the treatment period (Week 3) and 4 weeks after end of treatment (Week 7) compared to placebo. The key performance evaluations included: tinnitus handicap inventory (THI), questionnaire on well-being, and tinnitus annoyance and tinnitus related symptoms VAS including hyperacusis.
• To assess the performance of the active (biomodulator) patch compared to placebo on the proportion responders at Week 7. Responders were defined as patients at Week 7 having a decrease from baseline of at least 2 points in the tinnitus annoyance VAS.
Inclusion criteria
• Male or female adults > 18 years of age.
• Patients must have signed an informed consent document indicating that they understand the purpose of and procedures required for the clinical investigation and are willing to participate in the clinical investigation.
• Patients who have suffered from tinnitus for ≥ 4 weeks before investigation starts.
• Manifested tinnitus score of 3 or above tinnitus annoyance scale (VAS 0-10).
Exclusion criteria
• Pregnant or breast-feeding or planning to become pregnant or breast-feed during the clinical investigation.
• History of malignancy or other serious medical condition within 5 years before screening.
• Serious skin disease.
• Ongoing serious life event.
• Simultaneous or previous (within 30 days prior to investigation start) participation in a clinical investigation using experimental drugs or devices.
• Patients who have started treatment or made changes in treatment with drugs known to influence tinnitus within 6 weeks before investigation starts.
• Patients with treated or untreated hypertension.
• Other tinnitus treatment within 6 weeks before investigation start.
• Previous use of the biomodulator patch.
• Known allergy or sensitivity to any of the compounds in the biomodulator or the placebo patches.
• Any condition that in the opinion of the investigator would make participation not in the best interest of the patient, or could prevent, limit, or confound the protocol-specified assessments.
• Patients with history of general pain disorder and patients using pain relief drugs on a regular basis.
Assessment of performance
Overall, performance evaluations included: tinnitus annoyance VAS (primary variable), THI, questionnaire on well-being, tinnitus related symptoms VAS including hyperacusis and proportion of responders. Safety evaluations included comparing the adverse events (AEs) and adverse device effects (ADEs) between patients randomized to active and placebo patches.
Visual analogue scale
The visual analogue scale (VAS) was used for measuring tinnitus annoyance, hyperacusis annoyance and tinnitus related symptoms. VAS measurements are valid and considered effective measurements for capturing reductions in tinnitus severity in patients with chronic tinnitus [31] . The patients were instructed to mark on the scale from 0 to 10 what best described their current annoyance, where 0 corresponds to “doesn’t annoy me at all” and 10 corresponds to “unbearably annoying”. Tinnitus annoyance, hyperacusis annoyance and tinnitus related symptoms VAS assessments were performed at the baseline/randomization visit (Day 1), at the end of treatment visit (Week 3), and at the 4 weeks posttreatment telephone contact visit (Week 7).
Tinnitus handicap inventory
The tinnitus handicap inventory (THI) is a validated 25-item self-rating instrument resulting in a total score between 0 and 100 points. Each item can yield a score of 0, 2 or 4, according to the following: “not affected” (0 points), “sometimes affected” (2 points) and “always affected” (4 points). THI includes items concerning general tinnitus severity, QoL and psychological aspects of tinnitus [32] . This assessment was performed at baseline/randomization visit (Day 1), at the end of treatment visit (Week 3), and at the 4 weeks post-treatment telephone contact visit (Week 7). The total score was calculated using the mean of all available item scores times 25 implying that missing item scores were replaced by the mean of all available item scores. If more than 10 item scores were missing the total score was defined as missing.
Questionnaire on well-being
The questionnaire on well-being is a validated 18-item self-rating instrument resulting in a total score between 0 and 72 points. Each item can yield a score of 0, 1, 2, 3 or 4 points, according to the following: “never” (0 points) to “very often” (4 points), on questions starting with “To what extent during the past week have you…” [33] . The questionnaire on well-being includes different positive general feelings during the past week including today. It was performed at baseline/randomization visit (Day 1), at the end of treatment visit (Week 3), and at the 4 weeks post-treatment telephone contact visit (Week 7). The total score was calculated using the mean of all available item scores times 18 implying that missing item scores were replaced by the mean of all available item scores. If more than 10 item scores were missing the total score was defined as missing.
Study rationale minimal clinical important difference (MCID) for responders
Currently, there are no objective measurements for the assessment of subjective tinnitus. Different patient self-rated questionnaires are widely used both in trials and clinical practice and there is no consensus on which one to select when assessing improvements in tinnitus.
VAS has been used in psychological assessment since the early 20th century for a wide variety of healthrelated constructs including pain, mood and QoL. VAS is brief, simple to use and minimal in terms of respondent burden. The tinnitus annoyance VAS is a very important tool for assessing improvement in tinnitus. However, in general, there is no consensus on what is considered as a minimum clinically important difference (MCID) and which methods should be used in estimating the MCID [34] . The MCID concept was proposed in 1989 to consolidate what outcomes in clinical trials really matter for patients [35] . From an individual perspective, in patients suffering from tinnitus, any decrease in points in VAS can be considered as a personal relief. In comparison with the symptom pain, the MCID in pain VAS ranges from 0.8 to 4 points as per a recent thorough review [36] . However, it appears as though that a clinically significant change in pain VAS is also related to the patient’s initial pain VAS score. This was concluded from a study where patients suffering from higher self-rated pain required a greater change (more than 2.5 points decrease), to achieve clinically significant changes in pain along the VAS [37] . This could also be applicable in tinnitus annoyance VAS when defining responders, with 2 points or more. Another entity that is closely associated with tinnitus is anxiety, where the MCID is estimated between 1 and 1.5 points along VAS [38] . To avoid random and non-clinically significant changes in patient’s individual estimations, changes of 2 or more points along VAS were considered as MCID in study and defined the responder population. The Tinnitus Research Initiative (TRI), has studied the MCID for the THI and identified a reduction in the THI score of 6 as the MICD [39] . Currently, there is no available data on MCID for the questionnaire on well-being, as this likely depends on the studied symptoms and/or conditions.
Safety measurements
Reporting of (Serious) Adverse Events and (Serious) Adverse Device Effects
All AEs and ADEs that occurred during the study, starting when the first patch was applied, were reported by the investigator in the eCRFs. AEs and ADEs were collected with a non-leading question at each clinic visit as well as by reporting events directly observed and spontaneously reported by the patient. The report included information on onset/stop date, nature, intensity, duration, relationship to use of the medical device (yes/ no, possibly), outcome, interventions and medications required. Possible serious adverse events (SAEs) were reported as outlined in the EU guideline MEDDEV 2.7/3 Clinical Investigations: Serious Adverse Event Reporting. This also included any SAE possibly caused by a serious adverse device effect (SADE).
Statistical analysis
All data was summarized by means of descriptive statistics. Continuous data was presented with the number of observations, mean value, standard deviation (SD), minimum (Min), median, and maximum (Max) value. Categorical data was presented as counts and percentages. Tests of proportions were done by the Fisher’s exact and Chi-Square test and continuous variables were tested by means of the Wilcoxon rank sum test. Changes within group over time were tested by means of the Wilcoxon signed rank test. All statistical analyses were performed using SAS® (Version 9.3 SAS Institute Inc., Cary, NC, USA).
Results
Investigational subjects
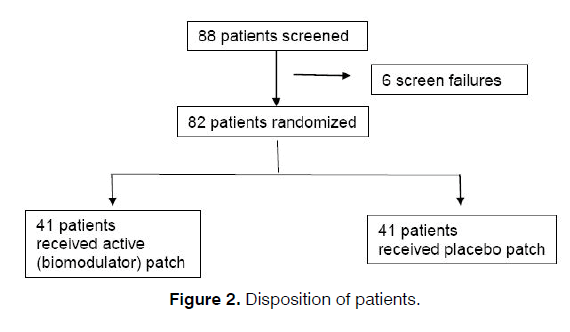
Patients suffering from tinnitus were recruited voluntary from both the ENT clinics patient database and from local advertisement. To show a statistically significant difference between the active and placebo group, a total of 78 patients were needed to be enrolled in this study. Eighty-eight (88) patients were enrolled, of which 82 patients were randomized: 41 patients received active patch and 41 patients received placebo treatments. All 82 patients completed the study with patch compliance and were included in the performance and efficacy analyses (Figure 2).
Baseline characteristics
A higher proportion of male than female patients were enrolled in both treatment groups. The age of all patients enrolled in the study ranged from 23 to 82 years. The mean age of patients in the placebo group was a bit higher than the mean age of patients in the active group (57.6 years in the placebo group compared to 53.5 years in the active group) (Table 2). Overall, the treatment groups were almost similar regarding demographics characteristics.
| ITT analysis set (N=82) | Treatment group | |
|---|---|---|
| Variable | Antinitus (N=41) | Placebo (N=41) |
| Sex, n (%) | ||
| Male | 25 (60.98) | 22 (53.66) |
| Female | 16 (39.02) | 19 (46.34) |
| Age, Years | ||
| n/n miss | 41/0 | 41/0 |
| Mean (SD) | 53.5 (11.07) | 57.6 (12.10) |
| Median | 53 | 56 |
| Min, Max | 26.0, 79.0 | 23.0, 82.0 |
Table 2. Summary of Baseline Demographics Characteristics.
At baseline, patients in the active group were suffering from tinnitus for mean of 13 years (range from 1 to 46 years) and in the placebo group for mean of 18 years (range from 0 to 56 years). Overall, 70% of patients had tinnitus in both ears, 15% of patients had tinnitus in the left ear and 15% of patients had tinnitus in the right ear. Forty-six percent (46%) of patients heard a beeping sound, 32% of patients heard a screeching noise and 10% of patient heard a hissing sound, and the rest with various experienced sounds. A total of 29% of patients in the active group has previously tried any treatment for tinnitus compared to 34% of patients in the placebo group (data not shown).
Performance results
Week 3
In summary, at Week 3, there were no statistically significant difference between the 2 treatment groups in either tinnitus annoyance VAS, THI or well-being. However, results tended to suggest that the active patch performed better than the placebo patch in all assessments, except the primary variable (tinnitus annoyance VAS), where there was no difference between baseline and Week 3. There was a statistically significant decrease (improvement) from baseline to Week 3 in THI in the active group (decrease of -8.0, p < 0.05) and in the placebo group (decrease of -4.3, p < 0.05). There was also an increase (improvement) from baseline to Week 3 in well-being in both the active group (increase of 3.0, p < 0.05) and in the placebo group (increase of 2.2, p < 0.05), although this was not statistically significant (p = 0.95) between the groups. In addition, was also a decrease (improvement) from baseline to Week 3 in hyperacusis VAS in both the active group (decrease of 1.0, p < 0.05) and in the placebo group (decrease of 0.7, p < 0.05), although this was not statistically significant between the groups.
Week 7
Tinnitus annoyance VAS
For both the active and placebo groups, the mean tinnitus annoyance VAS score decreased (improved) from baseline to Week 7. There was no statistically significant difference (p = 0.52) between the 2 groups at Week 7. However, the improvement in tinnitus annoyance VAS over time was statistically significant (p = 0.02) in active group (decrease in mean score of -0.6 over time). There was no statistically significant improvement (p = 0.18) in the placebo group (Table 3).
| Analysis set (N = 82) | Treatment group | |||
|---|---|---|---|---|
| Antinitus (N = 41) | Placebo (N = 41) | P-value* | ||
| Tinnitus annoyance VAS scale | ||||
| Baseline | Mean (SD) | 6.7 (1.66) | 6.3 (1.73) | |
| Week 7 | Mean (SD) | 6.1 (1.78) | 6.0 (1.95) | |
| Change | Mean (SD) | -0.6 (1.53) | -0.3 (1.34) | |
| P-value** | 0.0232* | 0.1769 | ||
| Wilcoxon Score | ||||
| Change*** | Mean rank score | 39.84 | 43.16 | 0.5189 |
**P-value based on Wilcoxon signed rank test
***Lower score means less annoyance
SD = Standard Deviation
Table 3. Change from baseline to Week 7 in the tinnitus annoyance VAS.
Tinnitus handicap inventory (THI)
For both the active and placebo groups, the mean THI decreased (improved) from baseline to Week 7. There was no statistically significant difference (p = 0.39) between the 2 groups at Week 7. However, the improvement in THI over time was statistically significant in both the active group (p < 0.001; decrease in mean score of -8.2 over time) and the placebo group (p = 0.002; decrease in mean score of -5.1 over time) (Table 4).
| Analysis set (N = 82) | Treatment group | |||
|---|---|---|---|---|
| Antinitus (N = 41) | Placebo (N = 41) | P-value* | ||
| Tinnitus Handicap Inventory | ||||
| Baseline | Mean (SD) | 37.6 (19.96) | 32.6 (20.59) | |
| Week 7 | Mean (SD) | 29.4 (17.07) | 27.6 (20.52) | |
| Change | Mean (SD) | -8.2 (11.45) | -5.1 (10.33) | |
| P-value** | <0.001* | 0.0017* | ||
| Wilcoxon Score | ||||
| Change*** | Mean rank score | 39.21 | 43.79 | 0.3846 |
**P-value based on Wilcoxon signed rank test
***Lower score means less annoyance
SD = Standard Deviation
Table 4. Change from baseline to Week 7 in the THI.
Questionnaire on well-being
For both the active and placebo groups, the mean well-being scored increased (improved) from baseline to Week 7. There was no statistically significant difference (p = 0.78) between the 2 groups at Week 7. In addition, the improvement of well-being over time was not statistically significant in either the active group (p = 0.12; increase in mean score of 1.8 over time) and the placebo group (p = 0.35) (Table 5).
| Analysis set (N = 82) | Treatment group | |||
|---|---|---|---|---|
| Antinitus (N = 41) | Placebo (N = 41) | P-value* | ||
| Well-Being | ||||
| Baseline | Mean (SD) | 49.1 (12.67) | 49.6 (11.61) | |
| Week 7 | Mean (SD) | 51.0 (12.86) | 49.8 (13.86) | |
| Change | Mean (SD) | 1.8 (6.79) | 0.2 (11.81) | |
| P-value** | 0.1223 | 0.3529 | ||
| Wilcoxon Score | ||||
| Change*** | Mean rank score | 42.24 | 40.76 | 0.7806 |
**P-value based on Wilcoxon signed rank test
***Higher score means more well-being
SD = Standard Deviation
Table 5. Change from baseline to Week 7 in the well-being questionnaire.
Hyperacusis rating VAS
For both the active and placebo groups, the mean hyperacusis VAS decreased (improved) from baseline to Week 7. There was no statistically significant difference (p = 0.23) between the 2 groups at Week 7. However, the improvement in hyperacusis VAS over time was statistically significant (p = 0.007) in the active group (decrease in mean score of -0.9 over time). There was no statistically significant improvement (p = 0.15) in the placebo group (decrease in mean score of -0.5 over time) (Table 6).
| Analysis set (N = 82) | Treatment group | |||
|---|---|---|---|---|
| Antinitus (N = 41) | Placebo (N = 41) | P-value* | ||
| VAS hyperacusis rating | ||||
| Baseline | Mean (SD) | 5.9 (2.71) | 5.4 (2.77) | |
| Week 7 | Mean (SD) | 5.0 (2.31) | 5.0 (2.36) | |
| Change | Mean (SD) | -0.9 (2.22) | -0.5 (1.98) | |
| P-value** | 0.0065* | 0.1537 | ||
| Wilcoxon Score | ||||
| Change*** | Mean rank score | 38.38 | 44.62 | 0.2282 |
**P-value based on Wilcoxon signed rank test
***Lower score means less annoyance
SD = Standard Deviation
Table 6. Change from baseline to Week 7 in the hyperacusis rating VAS.
Response at Week 7 in the tinnitus annoyance VAS
Responders, at Week 7, were defined as patients having a decrease from baseline of at least 2 points in the tinnitus annoyance VAS. At Week 7, there was a statistically significant (p < 0.05) higher proportion of responders in the active group (29.3% of patients with improvement) compared to the placebo group (9.8% of patients with improvements). There was also a statistically significant (p < 0.05) lower proportion of patients who had no change in VAS scores in the active group (61.0% of patients with no change) compared to the placebo group (82.9% of patients with no change). The number of patients showing a worse change were small in both groups and with no significant difference between the groups (Table 7).
| Treatment group | |||||
|---|---|---|---|---|---|
| Analysis set (N = 82) | Antinitus (N = 41) | Placebo (N = 41) | |||
| Tinnitus annoyance VAS scale | % | n | % | n | P-value |
| Improvement | 29.27 | 12 | 9.76 | 4 | 0.0488* |
| No change | 60.98 | 25 | 82.93 | 34 | 0.0479* |
| Worse | 9.76 | 4 | 7.32 | 3 | 1.0000 |
No change - defined as the same score or one point lower or one point higher
Worse - defined as an increase in score of at least two points
P-value based on Fisher’s exact test
Table 7. Response at Week 7 in the tinnitus annoyance VAS.
Tinnitus annoyance scale Responders
Analysis on the responder population (Table 7) showed that for both the active and placebo groups, the mean THI decreased (improved) from baseline to Week 7. There was no statistically significant difference (p = 0.16) between the 2 responder groups at Week 7. However, the improvement in THI over time was statistically significant (p = 0.0005) in the active responder group with a clinical significant improvement (decrease in mean score of -16 over time). There was no statistically significant improvement (p = 0.12) in the placebo responder group (decrease in mean score of -8.0 over time) (Table 8).
| Responder analysis set (N = 16) | Treatment group | |||
|---|---|---|---|---|
| Antinitus (N = 12) | Placebo (N = 4) | P-value* | ||
| Tinnitus Handicap Inventory | ||||
| Baseline | Mean (SD) | 43.8 (16.61) | 28.5 (16.11) | |
| Week 7 | Mean (SD) | 27.8 (11.74) | 20.5 (13.40) | |
| Change | Mean (SD) | -16.0 (9.76) | -8.0 (3.27) | |
| P-value** | 0.0005* | 0.1250 | ||
| Wilcoxon Score | ||||
| Change*** | Mean rank score | 7.50 | 11.50 | 0.1610 |
**P-value based on Wilcoxon signed rank test
***Lower score means less annoyance
SD= Standard Deviation
Table 8. Change from baseline to Week 7 in the THI VAS Responders.
Well-being questionnaire Responders
For the active in the responder group the well-being improved with mean 2.6 points but without statistical significance. The placebo group did on the contrary changed with mean -6.5 points but with no statistical significance. The difference between the groups was not statistically significant (Table 9).
| Responder analysis set (N = 16) | Treatment group | |||
|---|---|---|---|---|
| Antinitus (N = 12) | Placebo (N = 4) | P-value* | ||
| Well-being | ||||
| Baseline | Mean (SD) | 48.1 (15.58) | 54.8 (7.89) | |
| Week 7 | Mean (SD) | 50.7 (12.54) | 48.3 (20.01) | |
| Mean change | Mean (SD) | 2.6 (8.62) | -6.5 (21.46) | |
| P-value** | 0.2715 | 0.8750 | ||
| Wilcoxon Score | ||||
| Mean change*** | Mean rank score | 9.00 | 7.00 | 0.5042 |
**P-value based on Wilcoxon signed rank test
***Higher score means more well-being
SD= Standard Deviation
Table 9. Change from baseline to Week 7 in the well-being questionnaire VAS Responders.
Safety
The safety evaluation showed comparable results in the two treatment groups as measured by the collected adverse events data. No safety or tolerability concerns were identified. Local and reversible skin irritation were found in 24% of the patients in the active group and in 17% of the patients in the placebo group with no statistically significant difference between the groups.
Discussion
Based on today’s knowledge, the theory is that tinnitus is the consequence of failed suppression of processed sound stimuli and an unbalanced neuroanatomical homeostatic system.
The central hearing system is, by survival instinct, focused on listening and processing the sound. In consequence there is a relay that can either react to, or suppress, the sound. In reaction it should lead to beneficial, so the human can hear and act upon it or it can be suppressed and put to unawareness. When it fails to suppress, by failed sensory or stimuli controlled inhibition, the sound comes to awareness as tinnitus and is interpret as an existing sound that seeks an attention and action. However, the sound does not lead to any act of advantage for the human being and a negative feeling about it starts with a stressful audio mode. When other parts of the brain are involved like centres for emotions, attentions and memory the tinnitus can be persistent and hard to suppress. Fear might be the strongest reflexive feeling the human has and if the phantom sound of tinnitus interacts with the brain’s amygdala, tinnitus will be a threatening signal and fear will be the main outcome from it and the vicious circle of tinnitus will be hard to stop. Tinnitus is therefore a chaotic react-hearing process that does not lead to any act of advantage for the human being. One implication of this fact is that coherency seems be of importance of maintaining an equilibrium in this complex central sound processing system.
Previous clinical experience with the coherency biomodulator patch used in this study indicated that a large proportion of users show a delayed clinical onset of response within a month after the 3-week treatment period. Therefore, it was of value to study patient’s one-month post-treatment. The reason for the delay in response is likely to be found in the body adjustments of signal substances and neurotransmitters, taking time to calibrate, normalize and/or improve. This is commonly seen, for example, when treating depressions with modern selective serotonin re-uptake inhibitor (SSRI) anti-depressant.
In this study we found a significant larger group of tinnitus annoyance VAS responders in the active (biomodulator) group compared to placebo, 4 weeks after end of treatment (Week 7) with a 3:1 ratio. This was however not reflected in the measurements of THI and well-being even if we found a trend within the groups that active performed non-significantly better (THI decreased with 16 points and Well-being improved with 2.6) than placebo (THI decreased with 8 points and Well-being did not improve with -6.5). The changes within the groups were statistically significant for THI but not for Well-being. The reason for this is unknown but these quality-of-life questions might require a longer time of relief and followup to be shown statistically. The planned one-year followup might show results in that direction.
Water is essential for all biosystem and with the biomodulator creating coherency in water this might positively affect other body systems, not only the auditory system. However, our knowledge about how this work is little and needs to be further investigated.
We have previously seen patients responding positively to treatment with the patch even much later than 7 weeks, and may push for a longer follow-up time for future studies. The post-market follow-up performed for the biomodulator patch the recent years has also shown that patients with a failure to relief with the first 3-week treatment a second or even a third treatment round can be recommended for achieving sufficient subjective efficacy [40] .
This also arise questions about if the active (biomodulator) patch could be adjusted in different “doses” and also with treatment schedules that are longer or with planned repetition for better and more rapid results. All of these factors, should be considered in a future research study plan.
So how efficient can we anticipate that the biomodulator patch could be according to the results found in this study? One measurement, especially used in pharmacoeconomic studies, is the Number Needed to Treat (NNT) meaning how many patients we must treat to get one better/positive responder [41] . Even if it was developed to facilitate the practice of evidence-based medicine, it has its shortcomings; it is based on control conditions that do not really exist in standard practice, especially when it includes intensive clinical management as for psychotherapy and similar [42] .
Roughly the lower the NNT is the better the treatment will be. The ideal NNT is 1, where everyone improves with treatment and no one improves with control/ placebo. In this study, with the definition of responders used, the NNT was 5. To put this into perspective, we can compare this to NNT = 11 for the treatment with systemic corticosteroids for Bell's Palsy or NNT between 6 to 10 for different antidepressants treating major depressive disorder [43, 44] . Taken under consideration the low frequency and mildness of the side effects of the Antinitus patch shown in this and the previous study of the patch [26] the NNT of 5 seems even more convincing in the decisionmaking approach for tinnitus sufferers.
Tinnitus is associated with significant healthcare costs worldwide. In the USA it was estimated to be around 660 USD per patient per year, in the Netherlands mean annual tinnitus-related healthcare cost was estimated to be 1544 Euro per patient, and in a recent study in the UK the average cost per patient per year was estimated to be UK 717 GBP [45-47] .
Not only because of the suffering tinnitus cause for the individuals but also because of its time-consuming and economic burden for the healthcare system it can be of large advantage to having a safe and sufficient relief alternative. An easy reachable consumer product could have many benefits, especially for those who have mild to moderate problems with tinnitus. We know that even a small but significant effect would have an enormous therapeutic impact [48] .
Conclusion
The patch acts as a steady state coherent biomodulator and could have a resetting effect on the auditory system for downregulating tinnitus awareness.
In summary, the patch was safe and well-tolerated and showed efficacy with significant difference between the active and placebo groups at Week 7 (4 weeks after end of the treatment period). The active patch group had 30% responders (i.e. decrease from baseline in at least 2 points on the tinnitus annoyance VAS) compared to 10% of responders in the placebo group. There was no significant difference in performance between the active (biomodulator) and placebo groups directly at the end of treatment (Week 3).
Tinnitus is a global and common problem with a complex central origin and a lack of effective, nonexpensive, and risk-free treatments that are not time consuming. There is a need for devices that can at least relief tinnitus to some degree. The patch is a medical device class I with a unique steady state coherent biomodulation action that in a cost-risk-benefit rationale according to this study can be very reasonable to recommend.
References
- Venters RS. Subjective tinnitus or tinnitus aurium. Proc R Soc Med. 1953;46(10):825-9.
- Shulman A, Goldstein B. Pharmacotherapy for severe, disabling, subjective, idiopathic tinnitus: 2005-2006. Int Tinnitus J. 2006;12(2):161-71.
- Noreña AJ, Farley BJ. Tinnitus-related neural activity: Theories of generation, propagation, and centralization. Hear Res. 2013; 295:161-71.
- Tunkel DE, Bauer CA, Sun GH, Rosenfeld RM, Chandrasekhar SS, Cunningham ER Jr, et al. Clinical practice guideline: Tinnitus. Otolaryngol Head Neck Surg. 2014;151(Suppl 2):S1-40.
- Azevedo AA, Figueiredo RR, Elgoyhen AB, Langguth B, Penido NO, Schlee W. Tinnitus treatment with oxytocin: A Pilot Study. Front Neurol. 2017;8:494.
- Staecker H, Morelock M, Kramer T, Chrbolka P, Ahn JH, Meyer T. Safety of repeated-dose intratympanic injections with am-101 in acute inner ear tinnitus. Otolaryngol Head Neck Surg. 2017;157(3):478-87.
- Kreuzer PM, Landgrebe M, Resch M, Husser O, Schecklmann M, Geisreiter F, et al. Feasibility, safety and efficacy of transcutaneous vagus nerve stimulation in chronic tinnitus: An open pilot study. Brain Stimul. 2014;7(5):740-7.
- Martinez-Devesa P, Perera R, Theodoulou M, Waddell A. Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev. 2010:CD005233.
- Phillips JS, McFerran D. Tinnitus Retraining Therapy (TRT) for tinnitus. Cochrane Database Syst Rev. 2010:CD007330.
- Searchfield GD, Durai M, Linford T. A State-of-the-Art Review: Personalization of Tinnitus Sound Therapy. Front Psychol. 2017;8:1599.
- Stein A, Wunderlich R, Lau P, Engell A, Wollbrink A, Shaykevich A, et al. Clinical trial on tonal tinnitus with tailor-made notched music training. BMC Neurol. 2016;16:38.
- Hobson J, Chisholm E, El Refaie A. Sound therapy (masking) in the management of tinnitus in adults. Cochrane Database Syst Rev. 2012:CD006371.
- Meng Z, Liu S, Zheng Y, Phillips JS. Repetitive transcranial magnetic stimulation for tinnitus. Cochrane Database Syst Rev. 2011:CD007946.
- Hoare DJ, Edmondson-Jones M, Sereda M, Akeroyd MA, Hall D. Amplification with hearing aids for patients with tinnitus and co-existing hearing loss. Cochrane Database Syst Rev. 2014:CD010151.
- Bennett MH, Kertesz T, Perleth M, Yeung P, Lehm JP. Hyperbaric oxygen for idiopathic sudden sensorineural hearing loss and tinnitus. Cochrane Database Syst Rev. 2012:10:CD004739.
- Liu F, Han X, Li Y, Yu S. Acupuncture in the treatment of tinnitus: A systematic review and meta-analysis. Eur Arch Otorhinolaryngol. 2016;273(2):285-94.
- Dehkordi MA, Einolghozati S, Ghasemi SM, Abolbashari S, Meshkat M, Behzad H. Effect of low-level laser therapy in the treatment of cochlear tinnitus: A double-blind, placebo-controlled study. Ear Nose Throat J. 2015;94(1):32-6.
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. ProcNatlAcadSci U S A. 2001;98(2):676-82.
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. ProcNatlAcadSci U S A. 2003;100(1):253-8.
- Schlee W, Mueller N, Hartmann T, Keil J, Lorenz I, Weisz N. Mapping cortical hubs in tinnitus. BMC Biol. 2009;7:80.
- Elgoyhen AB, Langguth B, De Ridder D, Vanneste S. Tinnitus: Perspectives from human neuroimaging. Nat Rev Neurosci. 2015;16(10):632-42.
- Shulman A, Goldstein B. Quantitative electroencephalography: Preliminary report--tinnitus. Int Tinnitus J. 2002;8(2):77-86.
- Vanneste S, De Ridder D. Noninvasive and invasive neuromodulation for the treatment of tinnitus: An overview. Neuromodulation. 2012;15(4):350-60.
- Hoare DJ, Adjamian P, Sereda M. Electrical Stimulation of the Ear, Head, Cranial Nerve, or Cortex for the Treatment of Tinnitus: A Scoping Review. Neural Plast. 2016;5130503.
- Bono I, DelGiudice E, Gamberale, Henry M. Emergence of the coherent structure of liquid water. Water. 2012;4:510-32.
- Ahnblad P. Pilot Investigation of a Topographical Filter Dermal Patch in Patients with Tinnitus. Int Tinnitus J. 2017;21(1):7-13.
-
References
1Sickla ENT-center-Nacka, Stockholm - AC - Sweden
2Citysjukhuset +7, Lilla badhusgatan, Gothenburg, Sweden
Institution: Department of Otolaryngology, Rhinolaryngology and Laryngology Sickla ENT-center-Nacka, Stockholm - AC - Sweden
Send correspondence to:
Peter Ahnblad
Sickla ENT-center, Atlashuset Planiavägen 513134 Nacka. E-mail: peter@supramed.sePaper submitted to the ITJ-EM (Editorial Manager System) on November 25, 2017; and accepted on December 19, 2017.
Citation: Ahnblad P, Nordkvist A. A Randomized, Placebo-Controlled, Double-Blind, Parallel Groups Study Evaluating the Performance and Safety of a Steady State Coherent Biomodulator Patch in the Treatment of Subjective Tinnitus. Int Tinnitus J. 2017; 21(2): 157-167