The International Tinnitus Journal
Official Journal of the Neurootological and Equilibriometric Society
Official Journal of the Brazil Federal District Otorhinolaryngologist Society
ISSN: 0946-5448

Google scholar citation report
Citations : 12717
The International Tinnitus Journal received 12717 citations as per google scholar report
The International Tinnitus Journal peer review process verified at publons
Indexed In
- Excerpta Medica
- Scimago
- SCOPUS
- Publons
- EMBASE
- Google Scholar
- Euro Pub
- CAS Source Index (CASSI)
- Index Medicus
- Medline
- PubMed
- UGC
- EBSCO
Volume 19, Issue 1 / June 2014
Research Article Pages:68-76
Prevalence of hearing loss in newborns of mothers who had malaria and were treated with antimalaric drugs in pregnancy
Authors: Fernanda Soares Aurelio; Isis Pereira Dutra; Virginia Braz da Silva; Andre Luiz Lopes Sampaio; Carlos Augusto Costa Pires Oliveira
PDF
Abstract
Introduction: Gestational malaria is related to very bad perinatal outcomes and antimalarial drugs used during pregnancy can be ototoxic to the fetus. Objective: To determine the prevalence of hearing loss in newborns of mothers who had malaria and treated with antimalarial drugs during pregnancy. Materials and Methods: Cross-sectional study which involved 35 newborns. All underwent neonatal hearing screening with otoacoustic emissions combined with automatic auditory brainstem response. Those who failed were referred to audiological diagnosis with evoked otoacoustic emissions and brainstem auditory evoked potential associated with tympanometry (1000 Hz). Data were obtained through interviews with the mothers’ and analyzing the patient chart and the newborn, and underwent to statistical tests analysis of variance, equality of two proportions and Chi-square test (p = 0.05).
Results: The infection by Plasmodium vivax and chloroquine treatment with the first quarter prevailed; 88.6% (n = 31) newborns passed the hearing screening and 11.4% (n = 4) failed, of these, 50% (n = 2) attended for diagnosis, being diagnosed with hearing loss.
Conclusion: The prevalence of hearing loss in newborns of mothers who had vivax malaria and used chloroquine during pregnancy was 3%.
Keywords: antimalarial, hearing, infant, malaria, newborn, pregnancy
Introduction
Hearing is one of the noblest of senses since its main function is related to the acquisition and development of oral language [1,2]. In this respect, hearing is a prerequisite for language acquisition and development [3]. Furthermore, hearing is essential for interpersonal relationships and relationships with the environment [2]. Therefore, hearing impairment during the first years of life of a child can interfere with the acquisition and development of speech and language [3-5].
Hearing impairment is considered to be highly disabling due to its consequences on human communication, as well as its impact on cognitive and psychosocial development and the development of oral and written language [6]. Auditory sensory deprivation interferes with the ability to interpret speech sounds and results in economic and educational disadvantages [2]. Any type of hearing loss can compromise language, learning, cognitive development and social inclusion of the child [7], and it is therefore important that hearing impairment is detected as early as possible. Newborn hearing screening (NHS) continues to be the only strategy able to detect early hearing alterations that could interfere with the quality of life of an individual [5,8].
The occurrence of malaria during pregnancy, particularly in the last trimester, can be determinant for infant health, with implications for fetal or neonatal development. The malaria parasites can easily cross the placental barrier or are transmitted through maternal-fetal transfusion [9]. A study demonstrated that pregnant women infected with Plasmodium falciparum present clinical and laboratory alterations, such as altered hemoglobin, hematocrit, creatinine, urea, bilirubin, and glucose. The neonatal complications observed included abortion, prematurity and low birth weight [10].
In Brazil, the occurrence of malaria during pregnancy is common in endemic areas such as the states of Roraima, Rondônia, Pará, Mato Grosso, Acre, and Amazonas [11]. The latest survey regarding the proportion of positive slides in pregnant women from the municipality of Porto Velho, Rondônia (RO), was performed in 2010 and revealed 331 cases of malaria in rural and urban areas, including 48 cases caused by P. falciparum, 280 cases caused by P. vivax and three cases of mixed malaria (P. falciparum + P. vivax) [12].
In the municipality of Porto Velho (RO) malaria in pregnancy has been shown to be related to poor perinatal outcomes such as preterm birth, extremely low, very low and low birth weight, and death [13]. In a study conducted in the same municipality, two newborns of mothers who had malaria and used antimalarial drugs in pregnancy were diagnosed with mild unilateral sensorineural hearing loss [14].
In another study, 12 children born to mothers who had been treated with antimalarial drugs during pregnancy had sensorineural hearing loss. This hearing loss was irreversible in eight of these children, with seven presenting profound hearing loss. The treatment indicated for the mothers was chloroquine, chloroquine combined with quinine, and quinine combined with Fansidar. Antimalarial drugs can cause ototoxicity due to damage to the cochlea. Cochlear damage initially involves the outer hair cells, generally affecting high-frequency hearing. Tinnitus and vertigo are the most frequent symptoms associated with ototoxicity [15].
In view of the above considerations, the objective of the present study was to verify the prevalence of hearing loss in newborns of mothers who had malaria and treated with antimalarial drugs during pregnancy and to characterize the audiologic findings according to newborn gestational age, birth weight, risk factors for hearing loss, type of malaria infection, type of medication used, and gestational period of malaria occurrence and drug treatment. In addition, the hearing alterations were described according to the variables cited.
Material and Methods
A cross-sectional, quantitative, descriptive-analytical, observational study was conducted using a multifactorial approach. The study was approved by the Ethics Committee of Faculdade São Lucas (Permit Nº 147.583). The study was conducted at the maternity unit of a referral hospital for high-risk pregnancies in the capital of Rondônia. At this hospital, an NHS Program is provided by a private clinic accredited by the National Health System (Sistema Único de Saúde - SUS), where diagnosis and intervention are performed.
The data were collected between November 2012 and March 2013. All puerperal women received information about the objectives and methodology of the study and agreed to the procedures by signing the free informed consent form. The following eligibility criteria were established for formation of the sample: newborns of women who had malaria in pregnancy, newborns participating in the NHS Program, and newborns born or hospitalized in the maternity unit of the state referral hospital for high-risk pregnancies in Porto Velho (RO) whose mothers agreed to participate in the study by signing the free informed consent form. Excluded were infants born to mothers who were unable to report the occurrence and gestational period of malaria infection and infants born in other maternity units who were therefore not seen at the NHS service of the maternity unit where the study was conducted.
The number of women with malaria during pregnancy, who were admitted to the hospital during the period of data collection, was taken as the basis for sample size calculation. This number was 40. Adopting a level of significance of 95% and a sampling error of 6%, the estimated sample size was 35 newborns. It was not possible to include more subjects because the period scheduled was not sufficient to continue the process. The final sample consisted of 35 mothers who had malaria and used antimalarial drugs in pregnancy and their children.
The mean age of the mothers participating in the study was 23.1 years (range: 15 to 39 years). About 80% of the participants lived in the municipality of Porto Velho, 17.1% were from the State of Rondônia, and 2.9% from another state (Lábrea/AM). As for to educational level, 51.4% of the mothers had incomplete elementary school and 85.7% reported no occupation with a fixed monthly income. Fifteen of the newborns were girls and 20 were boys. The mean gestational age was 38-6/7 weeks (range: 30 to 41 weeks). The mean birth weight of the newborns was 3,194 g (range: 1,520 to 4,730 g).
A study flow chart elaborated by the researchers was used for data collection. Transient otoacoustic emissions (TOAE) and automated auditory brainstem responses (AABR) were measured with an Accuscreen instrument (Madsen). The diagnostic ABR test was performed with the Bio-logic Navigator Pro system. Disposable insert earphones and electrodes were used for the two instruments, as well as disposable gloves, gauze and 70% alcohol to clean the infant’s face and for fixation of the electrodes. Subjects who needed to complete the diagnostic phase were referred to the clinic responsible for the NHS program.
The study flow chart contains data regarding infant gender, date of birth, type of malaria infection, gestational period of infection, type of medications used, and presence of risk factors for hearing loss established by the Joint Committee on Infant Hearing (JCIH) [16] and by the Multiprofessional Committee on Auditory Health (Comitê Multiprofissional em Saúde Auditiva - COMUSA) [17]. The results obtained during hearing screening and diagnostic evaluation were recorded on the study flow chart. Personal data for subsequent contact were also recorded in case it was necessary to schedule a day and time for a new assessment during both the screening and diagnostic evaluation phases.
The puerperal women were contacted by the researcher while still in the maternity ward in order to investigate the occurrence of malaria during pregnancy. Women who confirmed the presence of the disease were invited to participate in the study and received information about the objective and importance of the study and the procedures used. Mothers who agreed to participate in the study signed two copies of the free informed consent form, one was given to them and the other was kept by the responsible researcher.
After signing the informed consent, the interview was conducted with the puerperal participants. When necessary, the records of the women and their newborns were accessed to complement the information if the mother could not answer the question or was in doubt.
The tests were performed in a quiet room in the presence of the mother or an accompanying person. For electrophysiological assessment of hearing, the infants remained lying in the crib or on the mother’s lap during natural non-sedated sleep.
First, the TOAE test was performed. For this test, a probe microphone with a latex bulb was introduced into the external auditory canal of the newborn and emissions were evoked with a broadband click at an intensity of 80 dB SPL. Infants presenting normal outer hair cell function obtained a “pass” result, which occurs automatically.
The second test was the AABR. For this test, the skin in the areas of electrode placement (high forehead and mastoids) was cleaned to permit adequate impedance. Insert earphones were used for sound stimulation. The infant received a “pass” result if he achieved normal results at least in the AABR. Infants who failed the test were directly referred for diagnostic evaluation, a procedure adopted by the program to prevent abandonment by the mothers, in addition to the fact that ABR is not affected by vernix which would justify retesting within 15 days.
The step of diagnostic evaluation was performed at the clinic responsible for the NHS program after hospital discharge. Diagnostic evaluation consisted of tympanometry, diagnostic TOAE, diagnostic ABR, and an appointment with the otorhinolaryngologist. Tympanometry, which investigates tympano-ossicular system function, excluding possible middle ear alterations, was performed using a 1000-Hz probe tone since it is more sensitive in identifying these alterations in infants aged 0 to 4 months [18].
After this procedure, the diagnostic ABR test was performed to quantify and identify hearing impairment.
Newborns who have had the electrophysiological threshold for tone bursts at 2000 Hz with bilateral 30 dBHL were considered patients with normal hearing and remained in auditory monitoring the NHS program up to 24 months.
For data analysis, the distribution of the occurrence of the type of infection and of the gestational period when malaria occurred was first determined, followed by the determination of the distribution of the type of antimalarial medication used for treatment and the gestational period during which the medication was administered, using the test for equality of two proportions. The NHS results were correlated with birth weight and gestational age by analysis of variance (ANOVA). The frequency of risk factors for hearing loss was analyzed by the test for equality of two proportions and the NHS results were correlated with variables such as type of malaria infection, gestational period when malaria occurred, type of antimalarial agent and gestational period when it was used. Risk factors were analyzed by the chi-squared test in order to determine the relationship between the findings and the variables mentioned. A level of significance of 5% was adopted for all tests (p ≤ 0.05).
Results
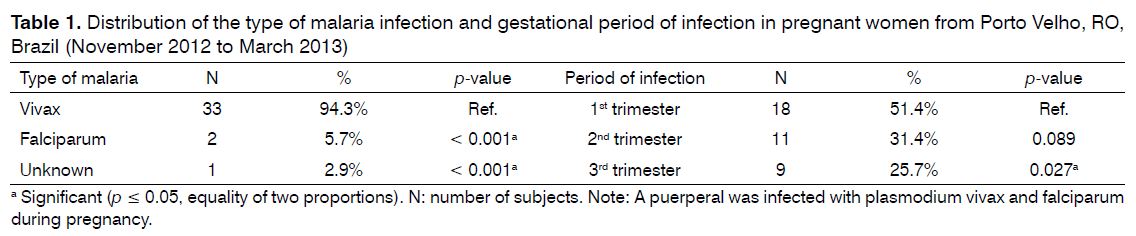
Plasmodium vivax was the most frequent species infecting women during pregnancy. The number of cases of P. vivax malaria was significantly higher than the number of cases of P. falciparum malaria. Infection occurred more frequently in the first trimester of gestation and this result was significant when compared to the third trimester (Table 1).
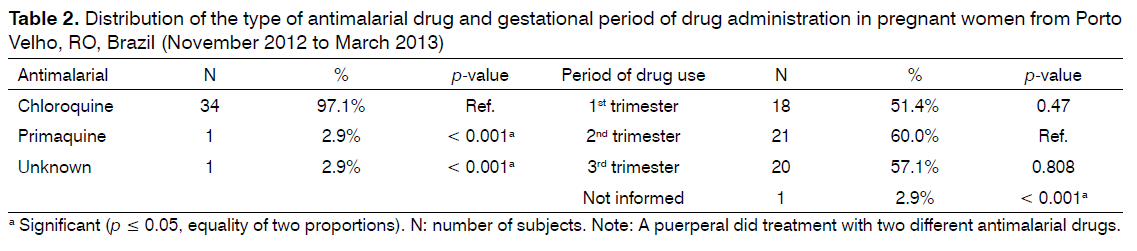
All women used antimalarial drugs during pregnancy. Chloroquine was the most frequently used drug. One of the women used two types of drugs during pregnancy. Antimalarial drug use was more frequent in the second trimester of gestation, but most participants reported use of the medication in more than one trimester (Table 2).
Thirty-one (88.6%) of the newborns studied passed the NHS (TOAE and AABR) and four (11.4%) failed the test, with this difference being significant (p < 0.001). Two of the four infants that failed the NHS and that were referred to the clinic responsible for the program for further tests abandoned the program.
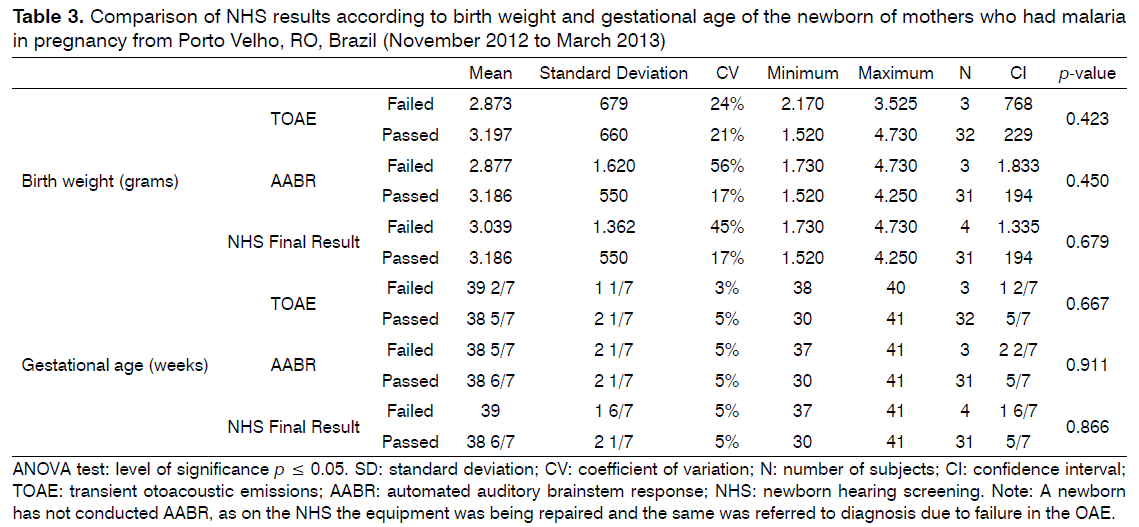
It was found that there is no relationship between the results of the NHS (pass/fail) with the variables weight of newborns at birth and gestational age (Table 3).
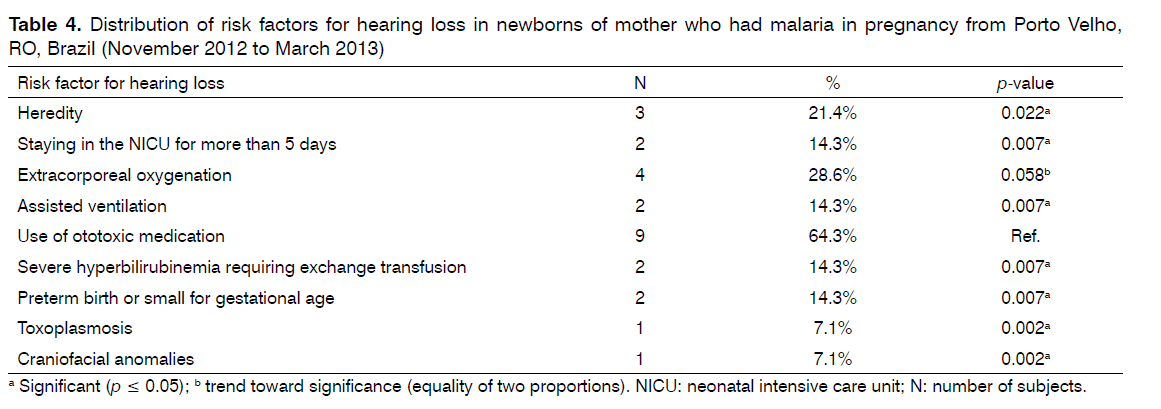
As for the risk factors for hearing loss found in association with the occurrence of malaria and the use of antimalarial drugs in pregnancy, the use of ototoxic medication by the newborn was the most frequent risk factor and this finding was significant when compared to the other risk factors, except for extracorporeal oxygenation (Table 4).
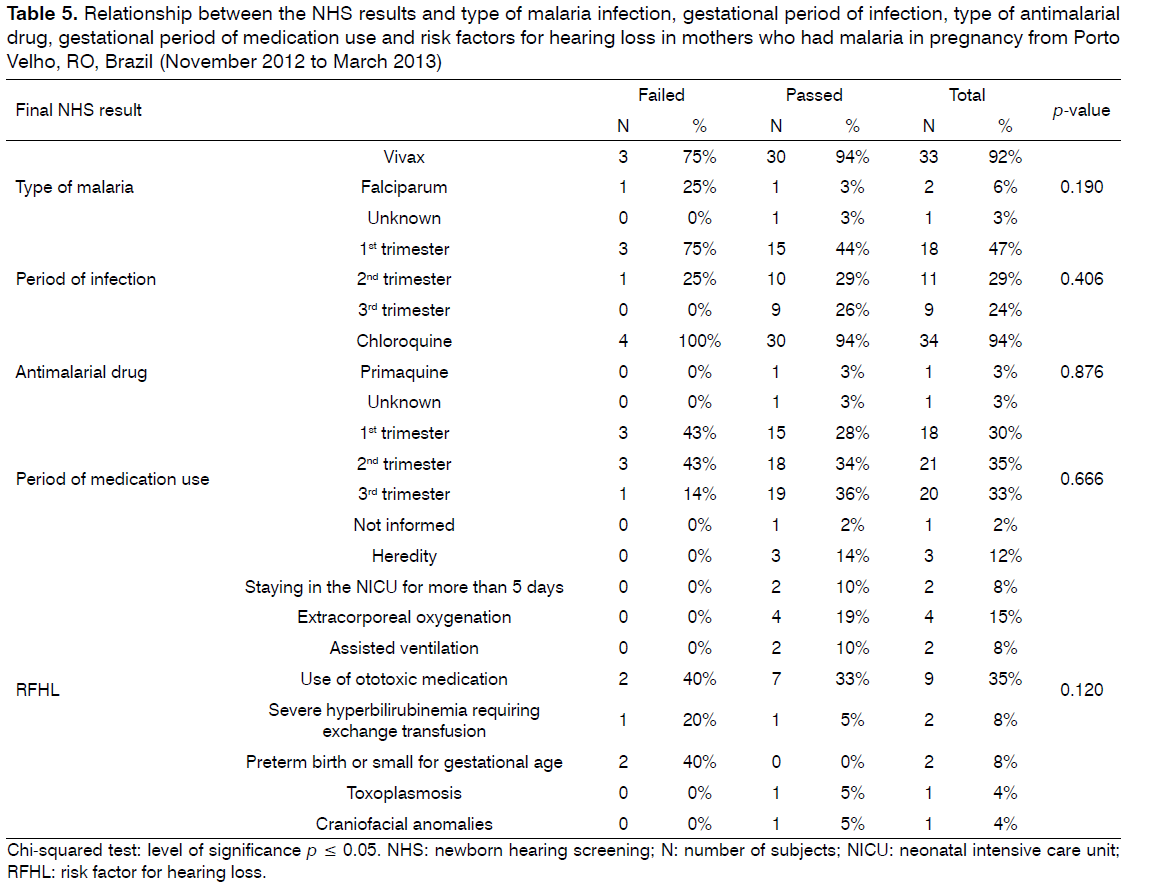
No relationship was observed between the NHS result and type of medication, risk factors for hearing loss, gestational period of infection, use of medication, or type of malaria infection (Table 5).
Table 5: Relationship between the NHS results and type of malaria infection, gestational period of infection, type of antimalarial drug, gestational period of medication use and risk factors for hearing loss in mothers who had malaria in pregnancy from Porto Velho, RO, Brazil (November 2012 to March 2013)
The prevalence of hearing loss in our sample was 3% (n = 1), taking into account that two newborns did not undergo the audiological diagnosis. The audiologic findings of this newborn included the bilateral presence of TOAE; absence of waves I, III and V at 90 dB in the click ABR test accompanied by the presence of cochlear microphonism, and tympanometry revealed normal mobility of the tympano-ossicular system. The otorhinolaryngologist requested imaging exams to evaluate possible involvement of the central auditory pathway. The results of these exams indicated the presence of bilateral impairment of the retrocochlear auditory pathways, findings compatible with the spectrum of auditory neuropathy.
This infant was born at term (37 weeks), but was small for gestational age. In addition to the fact that the mother had malaria and made use of antimalarial drugs during pregnancy, the newborn was exposed to ototoxic medication (gentamicin) for 9 days and required exchange transfusion due to hyperbilirubinemia. The Plasmodium species contracted by the mother during the first trimester of gestation was the vivax type. Chloroquine was used as antimalarial drug throughout gestation.
Discussion
In the present study, Plasmodium vivax was the most frequent species found in women during pregnancy, as also reported in other studies [10,13,19-21.]
The highest frequency of malaria was observed in the first trimester of gestation. This finding was statistically significant when compared to the occurrence of the disease in the third trimester. This result differs from that reported in another study [21] conducted in the same city, in which the distribution of malaria was homogenous across the three trimesters. This divergence may be explained by the difference in the period of data collection between the two studies, since the present study was conducted during the dry season (June to November), with the occurrence of malaria being related to climatic [22-24] and environmental factors and an increase in transmission being observed at the end of the rainy season [22].
The antimalarial drug most frequently used was chloroquine as recommended by the Brazilian Ministry of Health for the treatment of vivax malaria in pregnancy [25]. The use of primaquine is contraindicated for the treatment of pregnant women, irrespective of the Plasmodium species involved and gestational period, and during the breast-feeding period until 6 months [25]. In contrast, the World Health Organization recommends the use of primaquine under medical supervision [26]. However, this indication has become rare as observed in the present study and other studies in which pregnant women had malaria during pregnancy and were treated with chloroquine [14,27].
In a study conducted in Porto Velho (RO) on 66 newborns of mothers who used antimalarial drugs during pregnancy, three infants were born to mothers who were treated with primaquine and one was diagnosed with unilateral profound hearing loss [21]. This infant had no associated risk factor for hearing loss, suggesting that the medication administered possibly caused or potentiated the occurrence of hearing impairment.
As for the gestational period during which the antimalarial drug was used, no difference was observed between trimesters. However, the frequency of antimalarial drug use was higher in the second trimester, although the frequency of malaria occurrence was higher in the first trimester of gestation. In the present study, the participants reported the use of antimalarial drugs in more than one trimester, as recommended by the Brazilian Ministry of Health that indicate chloroquine as chemoprophylaxis against P. vivax. The drug should be administered for 3 days (25 mg/total dose) or 5 mg/kg per week for 3 months (maximum dose of 300 mg) and continuation of treatment after this period may be necessary in some cases [25].
In the present study, the number of “pass” results in NHS was higher than the number of “failed” results, in agreement with other studies conducted in the same municipality [14,21].
Two of the four newborns who failed the screening test and who were referred to the clinic responsible for the program for diagnostic evaluation did not attend the assessment. Despite an attempt to perform an active search, as routinely done, the mothers could not be contacted by telephone.
The socioeconomic conditions of the families who participate in the NHS program and the lack of knowledge of the consequences of hearing loss are some factors that could explain the evasion in the population studied, considering that the results of the study showed a low educational level of the women who mainly had incomplete elementary school. Similar reasons for evasion of NHS programs have been reported in other studies [28-30]. In this respect, it is important for the mother to have a minimum educational level, financial resources and harmony in social and marital relationships to seek the best health measures for her child [31].
No relationship was found between the NHS results and the variables weight at birth and gestational age, despite having knowledge that gestational malaria is related to very bad perinatal outcomes, including low birth weight [13] which could have enhanced the occurrence of hearing loss in newborns. The relationship between low birth weight and failed NHS and/or hearing loss has been shown in the literature [29,30]. Authors said that the lower the birth weight, the greater the chances of failure in the NHS [32,33], which was not observed in this study. No relationship between the NHS results and gestational age agrees with evidenced by other researchers [29].
The most frequent risk factor for hearing loss associated with malaria and antimalarial drug use in pregnancy was the use of ototoxic medication by the newborn, in agreement with other studies [21,34].
Urinary tract infection is common among young women and is the most frequent complication during pregnancy [30], especially among primiparous women [35]. The infant can be infected at the time of delivery, through hematogenic dissemination, or postpartum [36]. Once the infection is confirmed, antibiotic therapy is recommended [37].
The high level of bilirubin requiring blood transfusion was a risk factor for hearing loss in the infant diagnosed in the present study. Neonatal hyperbilirubinemia is toxic to the auditory pathways and central nervous system [29] and newborns submitted to exchange transfusion are considered to be at high risk for the development of auditory neuropathy [38].
Elevated serum levels of bilirubin are considered toxic to the auditory pathways and central nervous system [39]. In the latter, the yellow color is more intense in the subthalamic nucleus and globus pallidus and may be present in the hippocampus, geniculate bodies, vestibular, cochlear and oculomotor nuclei, cerebellum and spinal cord [40].
Hyperbilirubinemia is one of the risk factors for neonatal deafness and for the development of encephalopathies [39], since high levels of bilirubin can damage the auditory system at the peripheral level by altering cochlear hair cells and at the central level in the cochlear, olivary and inferior olivary nuclei [41]. Other studies have demonstrated a close relationship between hearing impairment and hyperbilirubinemia [5,39,40].
In addition to blood transfusion, the infant was exposed to ototoxic medication (gentamicin) which can cause different degrees of damage, with cochlear hair cells damage being typical [42]. A close relationship between hearing impairment and use of ototoxic medication has been reported for newborns [30,43,44].
The prevalence of hearing loss obtained in this study was more than evident in research conducted at the same hospita [5], and the value obtained could have been higher, since two newborns did not undergo the audiological diagnosis. The chance of these newborns have hearing loss is high, since the NHS was performed with use of combined technical (OAE and AABR), which reduces the chance of false positives. This result highlights the need to develop other studies with this population, but in larger samples.
In addition, it highlights the need for audiological follow these children in order to evaluate the occurrence of possible progressive alterations as demonstrated in another study which found 12 cases of hearing loss in children born to mothers who had malaria and used antimalarial drugs in pregnancy [15].
The main limitation of the present study, which is common in studies involving populations recruited from NHS programs, is failure to attend a visit for diagnostic evaluation. Active search was necessary in various cases of the present sample, but contacting the participants was unsuccessful in two cases. In addition, the rapid discharge of the participants, which is a major obstacle of NHS programs, may have contributed to the small sample size. Even if NHS is performed daily, including weekends and holidays, the large number of births per day in the maternity unit where the study was conducted leads to early discharge and the consequent loss of some newborns.
Conclusion
The prevalence of hearing loss in newborns of mothers who had malaria and treated with antimalarial drugs during pregnancy was 3% (n = 1), the mothers’ newborn diagnosed infected with Plasmodium vivax in the first trimester and treated with chloroquine throughout pregnancy. Audiological findings highlighted in this newborn indicate the presence of alteration in retrocochlear auditory pathways that are compatible with the auditory neuropathy spectrum.
In addition, it was observed that there is no relationship between the NHS findings with the variables weight of the newborn at birth, gestational age, type of medication, IRDA, pregnancy infection, medication use and infection type. The IRDA, concomitant with malaria and the use of antimalarial drugs during pregnancy, most common was the use of ototoxic medication by the newborn, but hyperbilirubinemia with exchange transfusion was one of the risk indicators found in newborns diagnosed with hearing loss.
References
- Hilú MRPB, Zeigelboim BS. O conhecimento, a valorização datriagemauditiva neonatal e a intervençãoprecoce da perdaauditiva. Rev CEFAC. 2007;9(4):563-70. DOI: http://dx.doi.org/10.1590/ S1516-18462007000400017
- Arakawa AM, Sitta EI, Caldana ML, Sales-Peres SHC. Análisedediferentesestudosepidemiológicosemaudiologiarealizados noBrasil. Rev CEFAC. 2011;13(1):152-8. DOI: http://dx.doi. org/10.1590/S1516-18462010005000089
- Gatto CI, Tochetto TM. Deficiênciaauditivainfantil: implicaçõesesoluções. Rev CEFAC. 2007;9(1):110-5. DOI: http://dx.doi. org/10.1590/S1516-18462007000100014
- Azevedo MF. Avaliaçãoaudiológica no primeiroano de vida. In:Lopes Filho OC. Tratado de fonoaudiologia. São Paulo: Roca; 1997. p.239-63.
- Botelho MS, Silva VB, ArrudaLda S, Kuniyoshi IC, Oliveira LL, OliveiraAS.Newborn hearing screening in the Limiar Clinic in Porto Velho - Rondônia. Braz J Otorhinolaryngol. 2010;76(5):605-10. DOI: http://dx.doi.org/10.1590/S1808-86942010000500012
- Freitas CD, Costa MJ. Hearing aids fitting process in users thatare seen in a federal public institution: part I--results and implications with the amplification device. Braz J Otorhinolaryngol. 2007;73(6):744-51.
- Vieira ABC, Macedo LR, Gonçalves DU. O diagnóstico da perdaauditivanainfância. Pediatria. 2007;29(1):43-9.
- GATANU - Grupo de Apoio à TriagemAuditiva Neonatal Universal[cited 2012 Jun 11]. Availavle from: http://www.gatanu.org
- Bardají A, Sigauque B, Sanz S, Maixenchs M, Ordi J, Aponte JJ,et al. Impact of malaria at the end of pregnancy on infant mortality and morbidity. J Infect Dis. 2011;203(5):691-9. PMID: 21199881 DOI: http://dx.doi.org/10.1093/infdis/jiq049
- Jarude R, Trindade R, Tavares-Neto J. Maláriaemgrávidas deumamaternidadepública de Rio Branco (Acre, Brasil). RBGO. 2003;25(3):149-54.
- Martins FSV, Castiñeiras TMPP, Pedro LGF. Malária. Centro de informaçãoemSaúdeparaviajantes CIVES. 21 de jun. 2011 [cited 2013 Apr 28]. Available from: http://www.cives.ufrj.br/informacao/ malaria/mal-iv.html.
- Brasil. Ministério da Saúde. SecretariaemVigilânciaemSaúde(SVS): proporção de lâminasparagrávidas - malária. Porto Velho: Sistema de Informação de VigilânciaEpidemiológica (SIVEP); 2010.
- Simões MCR. Prevalência de partosprematuros no Hospital deBase Dr.AryPinheiro (Porto Velho- RO) causadospelamalária durante a gestação no período de 2001 a 2003 emusuárias do SUS.[Masters Dissertation]. Brasília: Faculdade de Ciências da Saúde, Universidade de Brasília; 2006.
- Gama CFL, Souza RB. Avaliaçãoeletrofisiológica de recém--nascidos de mãesquefizeramuso de antimaláricosnagestação. [Term paper]. Porto Velho: Faculdade São Lucas; 2008.
- BrancoNeves MVSSC. Estudio de los efectosototóxicos en 725pacientestratados con antimaláricos en el hospital central de Maputo (Mozambique). [Thesis]. Barcelona: Facultat de Medicina, Departament de ciènciesmorfològiques (unitatd′anatomia; embriologia) da UniversitatAutònoma de Barcelona; 2004.
- American Academy of Pediatrics, Joint Committee on InfantHearing. Year 2007 position statement: Principles and guidelines for early hearing detection and intervention programs. Pediatrics. 2007;120(4):898-921. DOI: http://dx.doi.org/10.1542/peds.2007- 2333
- Lewis DR, Marone SA, Mendes BC, Cruz OL, Nóbrega Md. Multiprofessionalcommittee on auditory health: COMUSA. Braz J Otorhinolaryngol. 2010;76(1):121-8. DOI: http://dx.doi.org/10.1590/ S1808-86942010000100020
- Garcia MV, Azevedo MF, Testa JR. Accousticimmitance measuresin infants with 226 and 1000 hz probes: correlation with otoacoustic emissions and otoscopy examination. Braz J Otorhinolaryngol. 2009;75(1):80-9.
- Chagas ECS, Nascimento CT, Santana Filho FS, Bôtto-MenezesCH, Martinez-Espinosa FE.Maláriadurante a gravidez: efeitosobre ocurso da gestaçãonaregiãoamazônica. Rev PanamSalud Publica. 2009;26(3):203-08. DOI: http://dx.doi.org/10.1590/S1020- 49892009000900003
- Tobón-Castaño A, Solano MA, Sánchez LGA, Trujillo SB. Retardo nocrescimentointrauterino, baixo peso aonascer e prematuridadeem recém-nascidos de grávidas com malária, naColômbia. Rev Soc Bras Med Trop. 2011;44(3):364-70. DOI: http://dx.doi.org/10.1590/ S0037-86822011005000030
- Aurélio FS, Botelho MSN, Silva VB, Dutra IP, Sousa MA. Achadosaudiológicos de recém-nascidos de mãesquefizeramuso de antimaláricos no períodogestacional [Resumo n. P 005] In: Anais do 28º EncontroInternacional de Audiologia; 2013; Salvador, BA [anaiseletrônicos]. Salvador, BA;2013.[Cited 2013 May 18]. Available from: http://www.audiologiabrasil.org.br/eiasalvador2013/ anais/pos-ter.pdf
- Ribeiro JBM, Maia ABS, Rocha EJP, Ferreira MAV. PerfilEpidemiológicoeMicrometeorológico da MalárianaIlha de Cotijuba-PA. In: CongressoBrasileiro de Meteorologia. Fortaleza; 2004.
- Maciel FO, Lima e Silva RB, Souto RNP. Fatores de riscosassociadosàtransmissão de maláriahumana, emáreas de ressacas, nosbairros Novo Horizonte e Zerão, Macapá, Amapá, Brasil. Biota Amazônia. 2011;1(1):49-57. DOI: http://dx.doi.org/10.18561/2179- 5746/biotaamazonia.v1n1p49-57
- Amanajás JC, Nascimento GSL, Pereira IC, Braga CC. Associaçãoentreincidência de maláriaautóctone e precipitação no Estado do Amapá.Anais do 4º SimpósioInternacional de Climatologia. Brasil. João Pessoa; 2011.
- Brasil. Ministério da Saúde. Secretaria de Atenção à Saúde.DepartamentodeAçõesProgramáticasEstratégicas. Gestação de alto risco: manual técnico. 5a ed. Brasília: Ministério da Saúde; 2010.
- Word Health Organization. Guidelines for the treatment of malaria.2nd ed. Geneva: WHO; 2010.
- Amoran OE, Ariba AA, Iyaniwura CA. Determinants of intermittentpreventive treatment of malaria during pregnancy (IPTp) utilization in a rural town in Western Nigeria. Reprod Health. 2012;9:12. DOI: http://dx.doi.org/10.1186/1742-4755-9-12
- Alvarenga KF, Gadret JM, Araújo ES, Bevilacqua MC. Triagemauditiva neonatal: motivos da evasão das famílias no processo de detecçãoprecoce. Rev Soc Bras Fonoaudiol. 2012;17(3):241-7. DOI: http://dx.doi.org/10.1590/S1516-80342012000300002
- Onoda RM, Azevedo MF, Santos AM. Neonatal Hearing Screening:failures, hearing loss and risk indicators. Braz J Otorhinolaryngol. 2011;77(6):775-83. PMID: 22183285 DOI: http://dx.doi.org/10.1590/ S1808-86942011000600015
- Griz SMS, Silva ARA, Barbosa CP, Menezes DC, Curado NRPV,Silveira AK, et al. Indicadores de riscoparaperdaauditivaem neonatos e lactentesatendidosem um programa de triagemauditiva neonatal. Rev CEFAC. 2010;13(2):281-91. DOI: http://dx.doi. org/10.1590/S1516-18462010005000071
- Lima MLLT, Assis ABR, Mercês GB, Barros PF, Griz SMS. Triagemauditiva: perfilsocioeconômico de mãe. Rev CEFAC. 2010;10(2): 254-60.
- Pereira PKS, Martins AS, Vieira MR, Azevedo MF. Programa de triagemauditiva neonatal: associação entre perdaauditiva e fatores de risco. Pró-Fono. 2007;19(3):267-78. DOI: http://dx.doi.org/10.1590/ S0104-56872007000300005
- Tiensoli LO, Goulart LMHF, Resende LM, Colosimo EA. Triagemauditivaem um hospital público de Belo Horizonte, Minas Gerais, Brasil: deficiênciaauditiva e seusfatores de riscoemneonatos elactantes. Cad SaúdePública. 2007;23(6):1431-41. DOI: http:// dx.doi.org/10.1590/S0102-311X2007000600018
- Barreira-Nielsen C, FuturoNeto HA, Gattaz G. Processo de implantaçãodeProgramas de SaúdeAuditivaemduasmaternidades públicas. Rev Soc Bras Fonoaudiol. 2007;12(2):99-105. DOI: http:// dx.doi.org/10.1590/S1516-80342007000200006
- Duarte G, Marcolin AC, Gonçalves CV, Quintana SM, BerezowskiAT, Nogueira AA, et al. Infecçãourinárianagravidez: análise dos métodosparadiagnóstico e do tratamento. Rev Bras Ginecol Obstet. 2002;24(7):471-7. DOI: http://dx.doi.org/10.1590/S0100- 72032002000700007
- Branchini OAG. Infecçõeshospitalares do recém-nascido. In:Basseto MCA, Brock R, Wajnsztejn R. Neonatologia um convite á atuaçãofonoaudiológica. São Paulo: Lovise; 1998. p.147-53.
- Ramos IAPO. Infecções do tratourinário. In: Basseto MCA, Brock R,Wajnsztejn R. Neonatologia um convite à atuaçãofonoaudiológica. São Paulo: Lovise; 1998. p.159-63.
- Martinho AC, Lewis DR. Achadosaudiológicosemcrianças comhiperbilirrubinemia neonatal: emenfoquenaneuropatiaauditiva/ dessincroniaauditiva. DistúrbComun. 2005;17(2):183-90.
- Silva DP, Martins RH. Analysis of transient otoacoustic emissionsand brainstem evoked auditory potentials in neonates with hyperbilirubinemia. Braz J Otorhinolaryngol. 2009;75(3):381-6. DOI: http:// dx.doi.org/10.1590/S1808-86942009000300013
- Cianciarullo MA, Durante AS, Carvallo R, Voegels R, Takahashi G,Vaz FAC. Perdaauditiva neonatal associada a hiperbilirrubinemia pordeficiência de glicose-6-fosfato desidrogenase: relato de caso. Pediatria (São Paulo). 2005;27(2):126-32.
- Araújo MCK, Lichtig I, Couto MIV, Monteiro SRG, Ramos JLA, VazFAC. Sequelasauditivas e neuropsicomotorasemrecém-nascidos determoictéricostratadospelosmétodosconvencionais. Pediatria (São Paulo). 1997;19(2):102-9.
- Bricks LF, Wong A, Bouskela MAL, Ramos JL. A. Nefro e OtotoxicidadeporDrogas e AgentesFísicos no Período Neonatal - Atualização. Pediatria (São Paulo). 1994;16(3):120-8.
- Câmara MFS, Azevedo MF, Lima JWO, Sartorato EL. Efeito defármacosototóxicosnaaudição de recém-nascidos de alto risco. Rev Soc Bras Fonoaudiol. 2010;15(3):376-82. DOI: http://dx.doi. org/10.1590/S1516-80342010000300011
- Jornada ALM. Comparação das alteraçõesauditivasemrecém--nascidos da UTI neonatal expostos e nãoexpostosaantibióticos. [Masters Dissertation]. Porto Alegre: PontifíciaUniversidadeCatólica do Rio Grande do Sul; 2009.
References
1Mestrado em Distúrbios da Comunicação Humana - UFSM. Docente do Curso de Fonoaudiologia da Faculdade São Lucas - Porto Velho (RO). Doutoranda em Ciências da Saúde (UNB). Faculdade São Lucas/Universidade de Brasília, Porto Velho, RO, Brasil. E-mail: fernandaurelio@yahoo.com.br
2Graduada em Fonoaudiologia - Faculdade São Lucas - Porto Velho - RO - Brasil. E-mail: isispdutra@gmail.com
3Mestre em Ciências da Saúde pela Universidade de Brasília. Docente do Curso de Fonoaudiologia e Pós-graduação em Audiologia da Faculdade São Lucas. Porto Velho. - Faculdade São Lucas - Porto Velho - RO - Brasil. E-mail: virginia@saolucas.edu.br
4Doutorado em Ciências. Médico do Setor de Saúde Auditiva e Implante Coclear Universidade de Brasília - UnB, Professor Substituto Otorrinolaringologia Universidade de Brasília - UnB. - Universidade de Brasília - Brasília - DF - Brasil. E-mail: andremarjy@uol.com.br
5PhD Otorrinolaringologista. Professor e orientador do programa de pós-graduação em Ciências da Saúde e Ciências Médicas da Universidade de Brasília - UnB. Universidade de Brasília - Brasília - DF - Brasil. E-mail: cacpoliveira@brturbo.com.br
Institution: Faculdade São Lucas.
Send correspondence to:
Fernanda Soares Aurélio
Rua Alexandre Guimarães, 1927
Areal. Porto Velho - Rondônia. CEP: 78916-450
E-mail:fernandaurelio@yahoo.com.br
Paper submitted to the ITJ-SGP (Publishing Management System) on September 24, 2014; and accepted on July 6, 2015. cod. 173
Citation: Aurélio FS, Dutra ÍP, Silva VB, Sampaio ALL, Oliveira CACP. Prevalence of hearing loss in newborns of mothers who had malaria and were treated with antimalaric drugs in pregnancy. Int Tinnitus J. 2014;19(1):68-76