The International Tinnitus Journal
Official Journal of the Neurootological and Equilibriometric Society
Official Journal of the Brazil Federal District Otorhinolaryngologist Society
ISSN: 0946-5448

Google scholar citation report
Citations : 12717
The International Tinnitus Journal received 12717 citations as per google scholar report
The International Tinnitus Journal peer review process verified at publons
Indexed In
- Excerpta Medica
- Scimago
- SCOPUS
- Publons
- EMBASE
- Google Scholar
- Euro Pub
- CAS Source Index (CASSI)
- Index Medicus
- Medline
- PubMed
- UGC
- EBSCO
Volume 19, Issue 2 / December 2015
Research Article Pages:20-25
Risk of hearing alterations in newborns of mothers treated for malaria
Authors: Virginia Braz da Silva; Marceli Agostinho Sousa; Isabel Cristiane Kuniyoshi; Fernanda Soares Aurelio; Andre Luiz Lopes Sampaio; Carlos Augusto Costa Pires de Oliveira
PDF
Abstract
Introduction: The antimalarial drugs can cause irreversible sensorineural hearing loss, and chloroquine phosphate use can be ototoxic to the fetus. Objective: To compare the results of hearing screening in newborns of mothers treated for malaria in pregnancy with the results of newborns of mothers untreated and check for increased risk of hearing alterations in the group exposed to treatment.
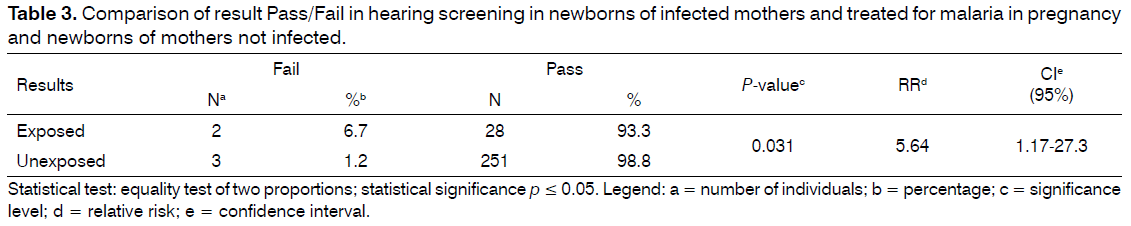
Method: Retrospective cohort study which involved 284 infants, 30 in the exposed group to malaria treatment and 254 in the unexposed group, matched for gestational age, birth weight and risk indicators for hearing. They underwent hearing screening by means of transient evoked otoacoustic emissions and/or auditory brainstem response automatic. The variables were collected in an interview with the parents in the health record and the screening database. Results: The prevalence of fail in exposed group (6.7%) was significant when compared with the unexposed group (1.2%). The risk of failing in the hearing screening in the exposed group was 5.64 (CI 1.17 to 27.3).
Conclusion: Newborns of mothers treated for malaria in pregnancy had a prevalence of fail in hearing screening at 6.7% and 5.64 higher chances to fail than newborns of untreated mothers.
Keywords: antimalarials, hearing, hearing loss, neonatal screening
Introduction
Hearing is one of the main individual means of contact with the outside world, playing a key role in their integration with society[1]. It allows the processing of acoustic events like speech, making possible to communicate with the expression of thinking[2]. Therefore, the hearing is in a precondition for the acquisition and development of language[3].
A child in acquiring your functions phase uses the language to organize your universe, understand the surrounding world, understand each other, transmit and abstract thoughts and feelings, interact with environment and acquire knowledge. In a child with hearing loss, the inability to communicate, can become an introverted individual, with problems of nervous origin, and end up isolating themselves from the surroundingworld because not understanding and not being understood[4].
There are several risks to which it is exposed the child’s hearing, when they were present in the pre, peri and post-natal. Some congenital infections have been described as high risk for hearing loss in childhood, such as toxoplasmosis, rubella, cytomegalovirus, herpes simplex and syphilis[5]. Malaria is an infection with risk of vertical transmission and important cause of mortality in the neonatal period and can lead to severe damage to the fetus[6].
Malaria is described as infectious disease caused by the plasmodium parasites, transmitted in nature by the bite of infected mosquitoes Anopheles type. It is considered one of the major public health problems because the plasmodium are found in an area which is home to nearly half the world’s population and when it occurs during pregnancy is responsible for broad spectrum of complications [7].
Currently in Brazil, malaria transmission is basically restricted to the Legal Amazon (Acre, Amapá, Amazonas, Maranhão, Mato Grosso, Pará, Rondônia, Roraima and Tocantins), and Rondônia is among the four states where it has the highest incidence[8].
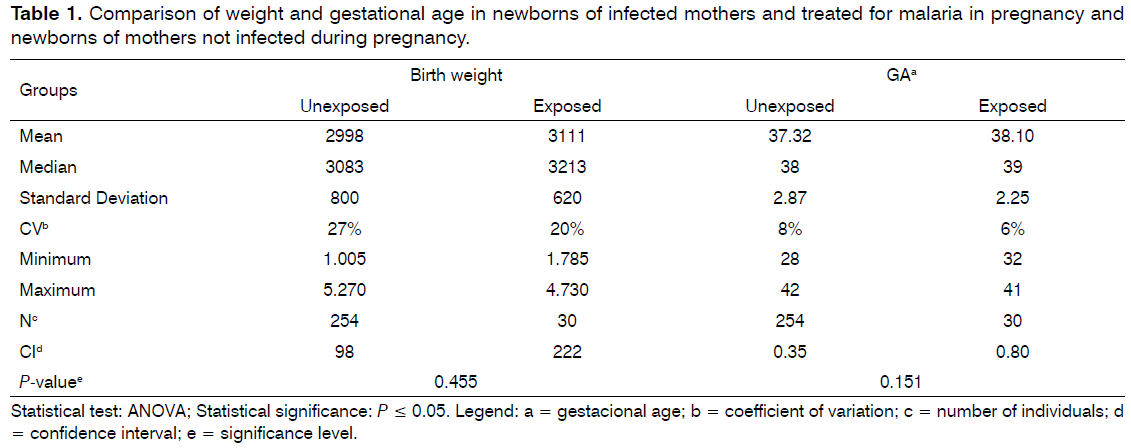
The effects in newborns of mothers infected with malaria in pregnancy have been insufficiently studied and so far it has been suggested that infection with Plasmodium Vivax is associated with low birth weight[9].
Another effect that is insufficiently studied is the ototoxic potential of the drugs used to treat malaria. These drugs can cause irreversible sensorineural hearing loss10 and it was shown that chloroquine phosphate use in the third quarter can be ototoxic to the fetus [11]. In other national studies, the authors identified cases of hearing loss in newborns of mothers infected with malaria during pregnancy[12,13].
Since hearing loss can be extremely harmful to children’s development and the lack of studies investigating the ototoxic effect of antimalarial drugs, it was necessary to conduct this study, which aimed to compare the results of newborn hearing screening (NHS) of newborns of infected mothers and treated for malaria in pregnancy with results in newborns from uninfected mothers.
Method
This study is part of a project entitled “Study of hearing in a neonatal hearing screening program in the Amazon region”, which was approved by the ethics committee on human research under the number 567/11. Parents whose newborns were part of this study, agreed to participate by signing the consent form.
This is an observational analytical study of retrospective cohort.
Data collection occurred in the NHS program conducted in a tertiary referral hospital in the care of pregnant women and newborns at high risk of Porto Velho (Rondonia), from February 2011 to February 2013.
The sample set to convenience or consecutively was estimated from the number of live births in the hospital where the study was conducted in the years 2011 and 2012, which was 6249 newborns. So we were using the sample calculation and adopting an error of 5% and 90% confidence level was reached on a minimum sample size of 260 newborns.
Newborns that participated in the study whose progenitors were infected with malaria during pregnancy and underwent treatment with antimalarial drugs (exposed group) and newborns of progenitors not infected by malaria in pregnancy who did not undergo treatment with antimalarial drugs (unexposed group). To be part of the study the newborn should have been submitted to the NHS and their parents or guardians should agree by signing the consent form.
To achieve the expected number in the sample were invited to participate in the study 335 parents and their newborns. However, 51 were excluded, 37 because they have not completed the NHS process, 13 due to the absence of the variables of interest (birth weight and/or gestational age) in the chart of the newborn, and a foreign mother newborn because of the difficulty for communication. Therefore, the sample consisted of 284 newborns, these 30 made up the exposed group and 254 non-exposed group.
The variables of interest in this study were collected in health records of mothers’ and/or interview with the mother or guardian, in their health records of newborn screening and database. In the chart and/ or interview with the mothers’ or guardians there was the history of infection and treatment for malaria during pregnancy, type of plasmodium, type of antimalarial drugs and trimester she started treatment. In addition, there was the occurrence of risk factors for hearing loss (RFHL)[5] such as toxoplasmosis infection, rubella, cytomegalovirus, herpes, syphilis and family history of hearing loss in childhood. In the health records of newborns, it was verified the gestational age and birth weight, as well as occurrence of RFHL[5], stay in neonatal intensive care units, use of ototoxic drugs, mechanical ventilation, extracorporeal membrane oxygenation, hyperbilirubinemia with serum level requiring exchange transfusion, syndrome associated with hearing impairment and craniofacial malformations. In the NHS database (HITRACK SOFTWARE 3.5) were verified the tests and their results (pass or fail).
The NHS program from hospital where the study was conducted following the protocol suggested by the Support Group Universal Newborn Hearing Screening [14]. Screening is performed by electrophysiological measurements with transient otoacoustic emissions (TOAE) and/or automatic auditory brainstem response (AABR). Newborns considered low risk (without RFHL) for hearing loss are submitted to NHS through TOAE and high risk (with RFHL) are screened using the combined technique of TOAE/AABR. In this program, as a criterion of the service itself, newborns of infected pregnant women for malaria during pregnancy, are also screened with use of the protocol for high-risk newborn hearing loss.
The equipment used by the NHS service to the research of TOAE and AABR is the automatic model portable MADSEN AccuScreen®, GN Otometrics. Initially, all newborns were tested for TOAE and following (after fixing the electrodes) the AABR was tested in the group exposed and unexposed in newborns identified as high risk for hearing loss in childhood, according to program protocol.
To perform the TOAE, the equipment has been calibrated by the manufacturer for automatic analysis of responses to the following parameters: evaluation by binomial statistics; stimulus type nonlinear click a sequence with 60 Hz speed; intensity of 70-84 dB SPL (45-60dB NS); frequency spectrum from 1.5 to 4.5 kHz; artifact lower than 20%. When parameters have been achieved the record “pass” was obtained.
In the record of the AABR, after checking the impedance of the electrodes (acceptable < 12 KOhms), the equipment made automatic analysis of responses with considering the following parameters: evaluation by binomial statistics; click stimulus of about 80Hz speed and intensity of 35 dB NS. When the optimal parameters were achieved, the equipment recorded “pass”.
The criteria for analyzing the results of the screening is to pass (no probability of hearing loss) and fail (there is probability of hearing loss and needs a full assessment). The low-risk newborns obtained aurally satisfying answers (pass) when showed the presence of TOAE bilaterally in the test or retest. High-risk newborns obtained aurally satisfying answers (pass) when showed, at least, the presence of AABR bilaterally. In the event that the result of the NHS was “fail”, newborns were referred for diagnostic and conduct evaluation.
For purposes of this study, it was analyzed data only include the detection (pass/fail) of hearing loss, ie, the NHS.
For the exposed group, it was found that 33.3% (n = 10) of the women were infected with malaria in the first trimester of pregnancy, 26.7% (n = 8) in the second, 30.0% (n = 9) in the third and 13.3% (n = 4) did not know their gestational period; 80% (n = 24) were infected with Plasmodium Vivax, 10% (n = 3) for Falciparum, 6.7% (n = 2) for both Plasmodium and 3.3% (n = 1) did not know the type of plasmodium. Finally, 66.7% (n = 20) were treated with chloroquine, 10% (n = 3) with primaquine, 6.7% (n = 2) Coartem, 3.3% (n = 1) Quinine and 16.6% (n = 5) did not know the medicine used in treatment.
After the formation of the groups and to ensure that they were matched on possible confounding variables (gestational age, birth weight and risk factors for hearing loss in childhood), they were compared for mean gestational age and birth weight through the ANOVA test and on the frequency of risk factors for hearing loss in childhood through the equality test of two proportions. It was observed that the pairing of the groups regarding gestational age and birth weight were no significant differences (P = 0.151 and 0.455, respectively). Besides having low coefficient of variation which showed that the sample is homogeneous (Table 1).
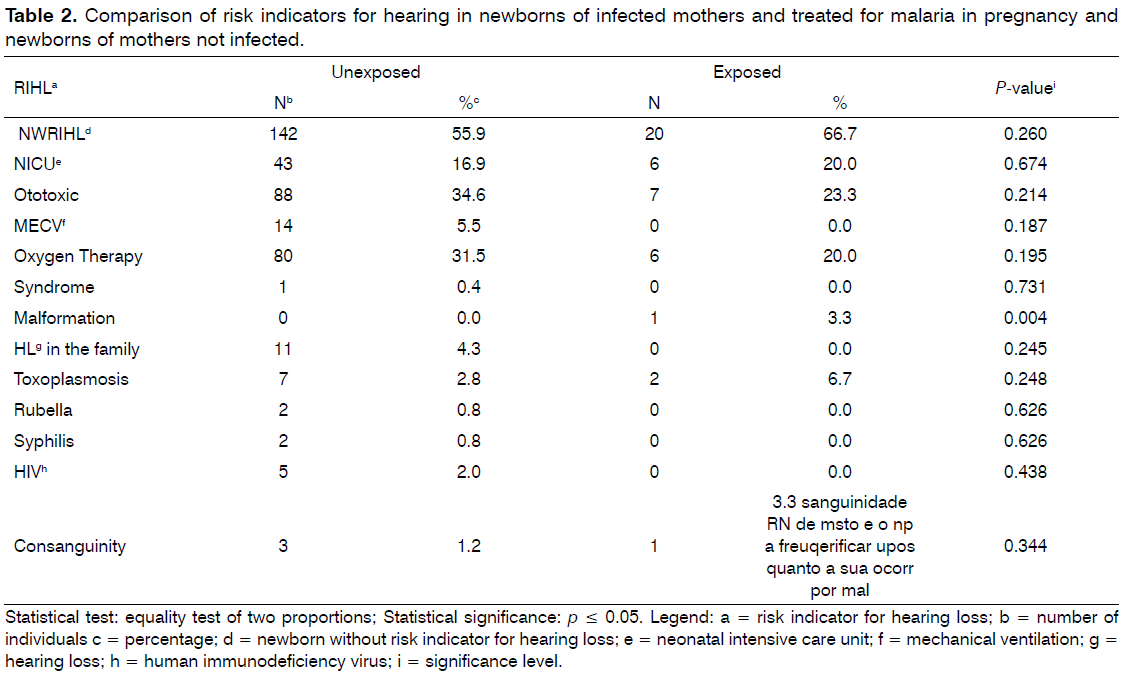
Also, significant difference was not detected between groups with RFHL, but it was identified a case of craniofacial malformations in the exposed group, significant compared with the group not exposed (Table 2). However, this particular case did not present altered results in the NHS.
To achieve the proposed objectives in this study, after the composition of the groups they were compared for NHS results. For this analysis we used the equality test of two proportions. Finally, it has been found the relative risk in exposed group of the NHS fails.
Statistical tests showed a 0.05 significance level (5%).
Results
The comparison between the result of the NHS (pass/fail) between the exposed and unexposed groups to malaria during pregnancy and their treatment, there was significant difference (P-value = 0.031) and the risk of fail in the exposed group was 5.64 (CI = 1.17 to 27.3) (Table 3).
Discussion
Symptoms of ototoxicity related to Chloroquine Phosphate are similar to those caused by aminoglycosides[12] and these can cause permanent hearing loss in later onset [5]. These statements and the fact that malaria during pregnancy associated with prematurity increases the chance of the child present alteration in the development [25] makes us conclude the need to monitor the hearing of this population with early identification of the occurrence of hearing loss and/or alteration in development hearing.
An important limitation to conduct this study was the low number of scientific publications related to the theme, besides the fact of not using methodologies recommended for the investigation of hearing loss in newborns and when used the correct methodology, the studies were merely descriptive.
Thus, it is concluded that newborns of mothers treated for malaria in pregnancy have prevalence of fails the hearing screening at 6.7% and 5.64 higher chances to fail in screening than newborns of untreated mothers. Considering the impact that hearing loss causes in child development and the high incidence of malaria worldwide, more studies are needed that rejected or proved the relation between the use of antimalarial drugs during pregnancy and the development of hearing alterations in newborns, in order to prevent or identify earlier this alterations.
References
- Colella-Santos MF, FrançozoMde F, do Couto CM, Lima MC, Tazinazzio TG, Castilho AM, et al. Audiological and genetics studies in high-risk infants. Braz J Otorhinolaryngol. 2011;77(6):784-90. PMID: 22183286 DOI: http://dx.doi.org/10.1590/S1808-86942011000600016
- Munhoz MSL, Caovila HH, Silva MLG, Ganança MM, eds. AudiologiaClínica. São Paulo: Atheneu; 2003. p.284.
- Gatto CI, Tochetto TM. Deficiênciaauditivainfantil: implicações e soluções. Rev CEFAC. 2007;9(1):110-5. DOI:http://dx.doi.org/10.1590/S1516-18462007000100014
- Roslyng-Jensen AMA. Importância do diagnósticoprecocenadeficiênciaauditiva. In: Lopes-Filho O, Org. Tratado de fonoaudiologia. São Paulo: Tecmedd; 2005. p.329-39.
- American Academy of Pediatrics, Joint Committee on Infant Hearing. Year 2007 position statement: Principles and guidelines for early hearing detection and intervention programs. Pediatrics. 2007;120(4):898-921. DOI: http://dx.doi.org/10.1542/peds.2007-2333
- Brock R, Martinez SMRC. Infecçõescongênitas. In: Bassetto MCA, Brock R, Wajnsztejn R. Neonatologia: um convite à atuaçãofonoaudiológica. São Paulo: Lovise; 1998. p.171-9.
- Jarude R, Trindade R, Tavares-Neto J. Maláriaemgrávidas de umamaternidadepública de Rio Branco (Acre, Brasil). Rev Bras Ginecol Obstet. 2003;25(3):149-54. DOI: http://dx.doi.org/10.1590/S0100-72032003000300002
- CIVES. Centro de InformaçãoemSaúdeparaViajantes [cited 2013 Jun 6]. Available from: http://www.cives.ufrj.br/informacao/malaria/mal-iv.html
- Tobón-Castaño A, Solano MA, Sánchez LGA, Trujillo SB. Retardo no crescimentointrauterino, baixo peso aonascer e prematuridadeemrecém-nascidos de grávidas com malária, naColômbia. Rev Soc Bras Med Trop. 2011;44(3):364-70. DOI:http://dx.doi.org/10.1590/S0037-86822011005000030
- Branco-Neves MVSSC. Estudio de los efectosototóxicos en 725 pacientestratados con antimaláricos en el hospital central de Maputo (Mozambique). [Doctoral thesis]. Barcelona: Facultat de MedicinaDepartament de ciènciesmorfològiques (unitat d' anatomia; embriologia) da UniversitatAutònoma de Barcelona; 2004.
- Figueredo MC, Aterino CCCT, Monteiro CV, Levy RA. Antimaláricos e Ototoxicidade. Rev Bras Reumatol. 2004;44(3):212-4.
- Kuniyoshi IC, Gama CFL, Souza RB, Bouchabki CB, Botelho MSN, Silva VB. Audição de recém-nascidos de mãesquefizeramuso de antimaláricosnagestação. 17º CongressoBrasileiro de Fonoaudiologia e 1º CongressoIbero-Americano de Fonoaudiologia. SociedadeBrasileira de Fonoaudiologia. Salvador (BA): 2009. p.2482.
- Aurélio FS, Botelho MSN, Silva VB, Dutra IP, Sousa MA. Achadosaudiológicos de recém-nascidos de mãesquefizeramuso de antimaláricos no períodogestacional [Resumo n. P 005] In: Anais do 28º EncontroInternacional de Audiologia; 2013; Salvador, BA.
- Gatanu. Grupo de apoio a triagemauditiva neonatal universal [Internet website] [cited 2013 Jun 8]. Available from: http://www.gatanu.org/secoes/programa-de-tratamento/itens/triagem/sub-itens/introducao-2
- Oliveira JAA, Canedo DM, Rossato M. Otoproteção das célulasciliadasauditivas contra aototoxicidade da amicacina. Rev Bras Otorrinolaringol. 2002;68(1):7-13. DOI: http://dx.doi.org/10.1590/S0034-72992002000100002
- Brasil. Guiaprático de tratamento da malária no Brasil. Ministério da Saúde, Secretaria de vigilânciaepidemiológica. Departamento de vigilânciaepidemiológica. Brasília; 2010. p.36. [cited 2015 Nov 25]. Available from: http://bvsms.saude.gov.br/bvs/publicacoes/guia_pratico_malaria.pdf
- Simões MCR. Prevalência de PartosPrematuros no Hospital de Base "Dr.AryPinheiro" (Porto Velho - RO) causadospormaláriadurante a gestação no período de 2001 a 2003 emusuárias do SUS. [Master's Degree dissertation]. Universidade de Brasília; 2006.
- Berni OS, Almeida EOC, Amado BCT, Almeida Filho N. Triagemauditiva neonatal universal: índice de efetividade no reteste de neonatos de um hospital da redepública de Campinas. Rev CEFAC. 2010;12(1):122-7. DOI: http://dx.doi.org/10.1590/S1516-18462009005000034
- Tomiasi AA, Duarte ST, Soria FS, Cunha JT, Cremonese AR, Cosmo BA. Acompanhamento de um programa de triagemauditiva neonatal universal desenvolvidonacidade de Cascavel-PR. Annals of 28o EncontroInternacional de Audiologia. Salvador; 2013. [cited 2015 Nov 25]. Available from: http://www.audiologiabrasil.org.br/eiasalvador2013/anais/poster.pdf
- Adam I, Idris HM, Elbashir MI. Quinine for chloroquine-resistant falciparum malaria in pregnant Sudanese women in the first trimester East Mediterr Health J. 2004;10(4-5):560-5.
- Adam I, Mirghani OA, Saed OK, Ahmed SM, Mohamadani AA, Ahmed HM, et al. Quinine therapy in severe Plasmodium falciparum malaria during pregnancy in Sudan. East Mediterr Health J. 2004;10(1-2):159-66.
- Lewis DR, Marone SA, Mendes BC, Cruz OL, Nóbrega Md. Multiprofessional committee on auditory health: COMUSA. Braz J Otorhinolaryngol. 2010;76(1):121-8. DOI: http://dx.doi.org/10.1590/S1808-86942010000100020
- Kuniyoshi IC, Aurélio FS, Dutra IP, Rodrigues LB, Silva VB. Relação da malárianagestação com alteraçõesauditivasnosneonatos. Anais do 29th EncontroInternacional de Audiologia. Florianópolis; 2014. p.431
- Uchôa NT, Procianoy RS, Lavinsky L, Sleifer P. Prevalência de perdaauditivaemrecém-nascidos de muitobaixo peso. J Pediatr (Rio J). 2003;79(2):123-8. DOI: http://dx.doi.org/10.1590/S0021-75572003000200006
- Simões MCR. Alterações do desenvolvimento de criançasprematurasnascidas de mães com malária no períodogestacional [Doctoral thesis]. Brasília: Universidade de Brasília, Faculdade de Ciências da Saúde; 2012.
References
1Post Graduation Program in Health Sciences - Universidade de Brasília - Brasília - DF - Brasil. E-mail: vbsfono@gmail.com / fernandaurelio@yahoo.com.br /cacpoliveira@brturbo.com.br
2Coordenação de Fonoaudiologia - Faculdade São Lucas - Porto Velho - RO - Brasil. E-mail: marceliagostinho.fono@gmail.com / isabelck@saolucas.edu.br /
3MD of the Department of Cochear Implant, Hospital Universitário de Brasília - Universidade de Brasília - Brasília - DF - Brasil. E-mail: andremarjy@uol.com.br
Institution: Universidade de Brasília
Send correspondence to:
Virgínia Braz da Silva
Avenida Engenheiro Anysio da Rocha Compasso
nº 4405, Apt 402, Bloco 4
Condomínio Brisas do Madeira, Porto Velho, RO
Brazil, Zip Code: 76804212
E-mail: vbsfono@gmail.comr
Paper submitted to the RBCMS-SGP (Publishing Management System) on June 25, 2015; and accepted on November 12, 2015. cod. 200
Citation: Silva VB, Sousa MA, Kuniyoshi IC, Aurélio FS, Sampaio ALL, Oliveira CACP. Risk of hearing alterations in newborns of mothers treated for malaria. Int Tinnitus J. 2015;19(2):20-25