The International Tinnitus Journal
Official Journal of the Neurootological and Equilibriometric Society
Official Journal of the Brazil Federal District Otorhinolaryngologist Society
ISSN: 0946-5448

Google scholar citation report
Citations : 12717
The International Tinnitus Journal received 12717 citations as per google scholar report
The International Tinnitus Journal peer review process verified at publons
Indexed In
- Excerpta Medica
- Scimago
- SCOPUS
- Publons
- EMBASE
- Google Scholar
- Euro Pub
- CAS Source Index (CASSI)
- Index Medicus
- Medline
- PubMed
- UGC
- EBSCO
Volume 26, Issue 2 / December 2022
Case Report Pages:115-121
10.5935/0946-5448.20220018
Case Reports on Effect of Intratympanic Steroid Injection Therapy on Late−Onset Hearing Loss in Congenital Cytomegalovirus Infection
Authors: Hideaki Sakata, Ken Hayashi, Eisuke Suganuma, Tsutomu Oishi
PDF
Abstract
Background: One of the conditions or symptoms caused by congenital cytomegalovirus (cCMV) infection is late-onset hearing loss. This report examines the cases of two children exhibiting late-onset hearing loss after cCMV infection who showed improvement in hearing after undergoing intratympanic steroid injection therapy (IST).
Cases: Case1 is girl aged 8 years and 10 months and case2 is girl aged 5 years and 1 month. Cytomegalovirus (CMV) was not detected in the blood or urine of either child at the time of hearing loss despite them having cCMV infection.
Findings: The hearing of both children improved as a result of IST on an outpatient basis. Case1 was given first session of IST in left ear immediately on the day of her visit and second session of IST in left ear 2 days later. Tendency for improvement in threshold on left side was observed (the differences were about 20 to 45 dB). Case2 was given a total of 2 sessions of IST on left ear, 3 days and 5 days after visiting the hospital. The test result of distortion product otoacoustic emissions changed from refer to pass. Tendency for improvement in threshold on left side was observed (the differences were about 5 to 25 dB).
Conclusions: Bearing in mind that late-onset hearing loss in patients with cCMV may be caused by other factors besides CMV, consideration of IST as a possible treatment option is proposed.
Keywords: Transtympanic infusion, Cytomegalovirus, Auditory brainstem response, Dexamethasone, Sudden deafness, Late-onset
hearing loss.
Introduction
Congenital Cytomegalovirus (cCMV) infection is estimated to affect 0.2 to 1% according to a recent review study [1]. And it is known to cause various signs/symptoms following mother-to-child transmission, including low birth weight, microcephaly, neurodevelopmental sequalae, chorioretinitis, and Sensorineural Hearing Loss (SNHL) [2,3].
The rate of no symptoms or signs at birth varies from study to study. Especially for SNHL, Yamaguchi et al. reported that congenital hearing loss occurs in approximately 1 out of 10 cases of symptomatic cCMV infection [4]. However, late-onset hearing loss is a major issue in that it is not detected by neonatal hearing screening tests and could be delayed in detection and delay in appropriate intervention.
Defined true incidence of late onset hearing loss as a percentage of children with asymptomatic infection who have a normal hearing at birth, asymptomatic cases alone putting prevalence at 1.4-17.6% [5,6]. There have been no previous reports of Intratympanic Steroid Injection Therapy (IST) for the treatment of late-onset hearing loss in cCMV patients. Since being reported as a therapy for Ménière’s disease by Sakata et al. [7], IST has gained recognition as a therapy for hearing loss, tinnitus and vertigo and has built up a proven track record of success [8,9].
This report focuses on the cases of two cCMV patients who presented with late-onset hearing loss and whose condition improved following IST on an outpatient basis.
Case Report
Case1: Gender and age: Girl aged 8 years and 10 months.
Information about Birth: Born at 33 weeks and 2 days (Caesarean section); head circumference 27.6cm; birth weight 1,295g; body length 39.0cm; managed 2 months in NICU due to low birth weight.
Results of Newborn Hearing Screening Test (Auditory Brainstem Response: Abr): Passed on both sides; blood and urine specimens taken at time of screening tested positive for Cytomegalovirus (CMV) (real-time PCR was performed a few days after birth). The umbilical cord dried blood DNA test was CMV positive.
No significant findings on MRI (at age of 10 months and at 2 years). CT scan of the head: microcephaly; calcification. No treatment for cCMV given. No particular abnormalities in subsequent motor development.
At 6 Years and 5 Months: Patient was diagnosed as SNHL in right ear in a medical checkup on enrolment in elementary school by school doctor. Patient consulted local ear and nose clinic but patient’s SNHL sign was missed and a “watch and wait” approach was adopted.
At 6 Years and 10 Months: Patient first visited the hospital after mother sensed that patient’s hearing in right ear had recently deteriorated. At that point, 3 weeks had already elapsed from the time mother sensed a deterioration in patient’s hearing. At the strong request of patient’s guardian, patient was prescribed prednisolone (20mg dose reduced gradually over 1 week), adenosine triphosphate disodium hydrate as oral medication, even though the therapeutic effect was expected to be low, but there was no therapeutic effect.
In addition, patient was given 4 sessions of IST in the right ear (4 days, 7 days, 11 days and 14 days after first visit to the hospital). However, there was no threshold change (91.25 dB given by the calculation called the one fourth methods). Patient’s hearing stabilized and periodic management of hearing commenced.
At 8 years and 10 months: Patient visited the hospital once again saying that hearing on left side had deteriorated 2 days before. A comparison with previous hearing test results revealed a loss of hearing in left ear (the differences were about 30 to 40 dB; figure 1). We ruled out conductive hearing loss by confirming inner ear findings under a microscope and tympanic membrane mobility by tympanometry. CMV: 1.0×102copies/mL in urine. Patient was given first session of IST in left ear immediately on the day of her visit. Patient was given second session of IST in left ear 2 days later. Tendency for improvement in threshold on left side was observed (the differences were about 20 to 45 dB). Patient confirmed improvement in hearing in left ear 3 days later (Figure 2). We followed this patient for 9 months with no change in hearing.
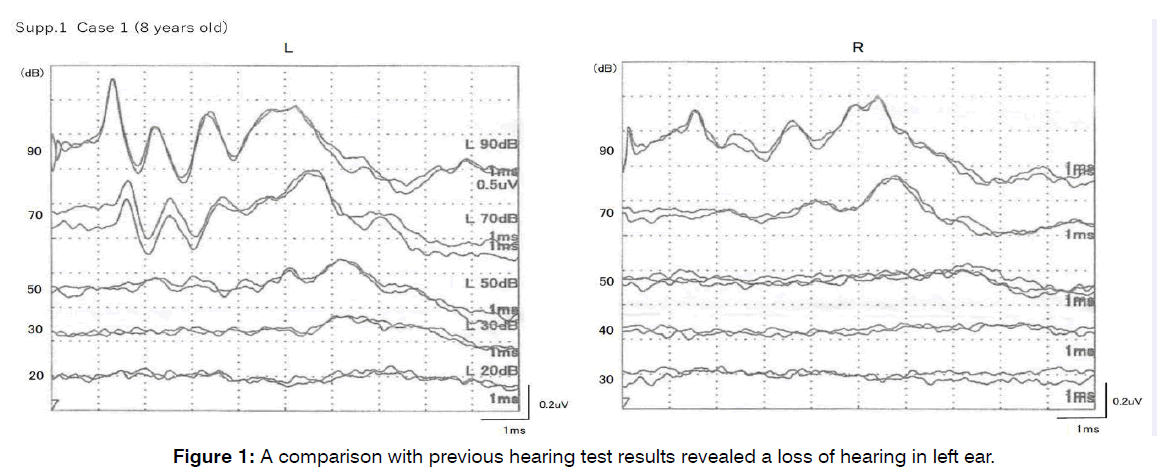
Figure 1: A comparison with previous hearing test results revealed a loss of hearing in left ear.
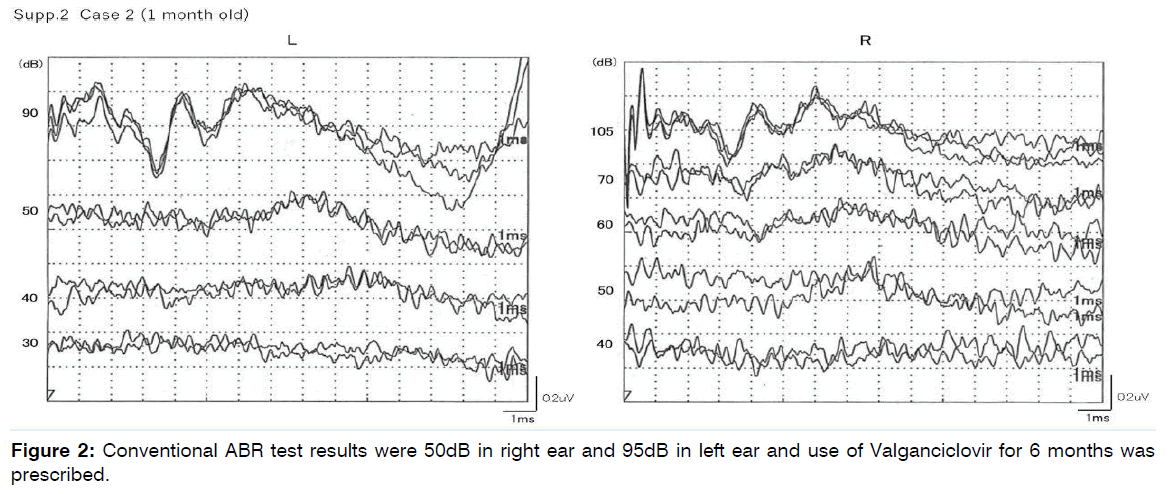
Figure 2: Conventional ABR test results were 50dB in right ear and 95dB in left ear and use of Valganciclovir for 6 months was prescribed.
Case 2: Age and gender: Girl aged 5 years and 1 month. Information about birth: Born at 39 weeks and 4 days (vaginal delivery); head circumference 31.8cm; birth weight 2,380g; body length 49.0cm.
Results of Newborn Hearing Screening Test: Passed on both sides; tested positive for CMV in blood and urine tests on the second day after birth (The viral load was unknown). The umbilical cord dried blood DNA test was CMV positive.
CT scan at 0 years and 6 months showed no calcification. MRI scan of the head at the age of three showed a white matter abnormality around the mild lateral ventricle, lateral ventricle enlargement, and irregular cerebriform.
At 1 Month: Conventional ABR test results were 50dB in right ear and 95dB in left ear and use of Valganciclovir for 6 months was prescribed (Figure 3).
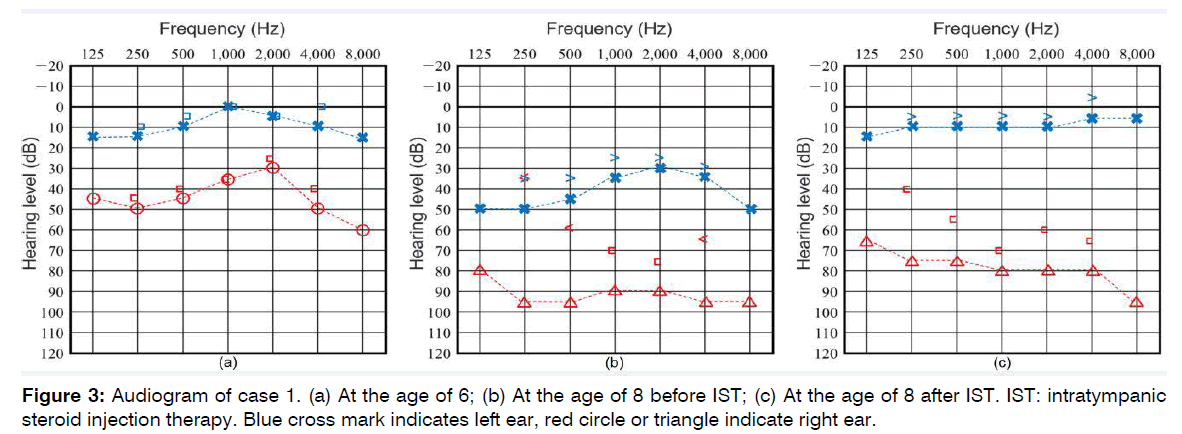
Figure 3: Audiogram of case 1. (a) At the age of 6; (b) At the age of 8 before IST; (c) At the age of 8 after IST. IST: intratympanic steroid injection therapy. Blue cross mark indicates left ear, red circle or triangle indicate right ear.
Table 1 shows age, ABR results and details of treatment. At approximately 4 years and 9 months: Progressive hearing loss on both sides. Another medical institution recommended cochlear implant because use of a hearing aid was not sufficiently effective.
| Age | ABR (Right) | ABR (Left) | Treatment |
|---|---|---|---|
| 0 year | 50dBnHL | 95dBnHL | Valganciclovir 6 months |
| 2 years and 11 months | 50dBnHL | 90dBnHL | - |
| 4 years and 7 months | 90dBnHL | 100dBnHL | Steroid 10 days |
Table 1: The course of case 2.
At 4 Years and 11 Months: Patient visited our outpatient clinic specializing in CMV for the first time. CMV: 1.0×102copies/mL.
Patient was given a total of 2 sessions of IST on left ear, 3 days and 5 days after visiting the hospital. At request of guardian, patient was given a total of 2 sessions of IST on the opposite right side 8 days and 12 days after visiting the hospital. Improvement in hearing was observed (Figure 4, 5). The test result of distortion product otoacoustic emissions changed from refer to pass. Tendency for improvement in threshold on left side was observed (the differences were about 5 to 25 dB). Examination by tympanometry showed bilateral type A and no otitis media. We followed the patient for 6 months and no problems. Patient is currently using a hearing aid on both sides and being actively monitored (“watch and wait” approach).
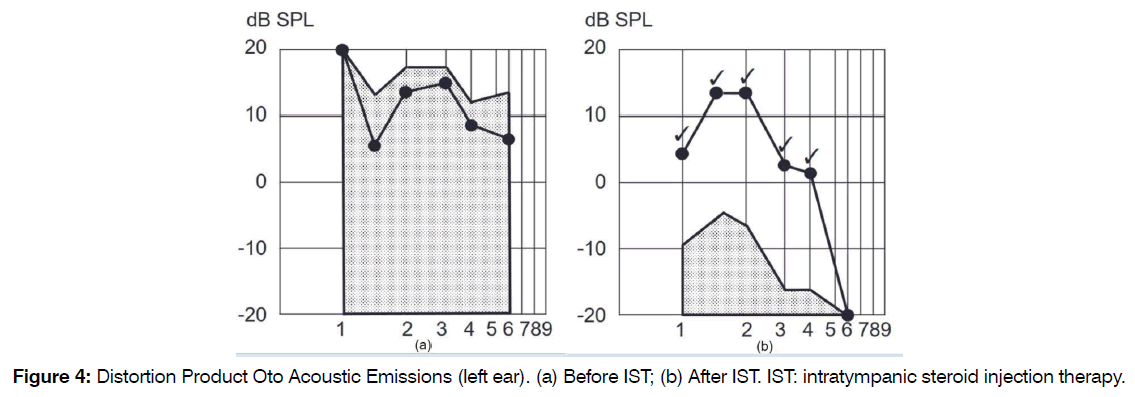
Figure 4: Distortion Product Oto Acoustic Emissions (left ear). (a) Before IST; (b) After IST. IST: intratympanic steroid injection therapy.
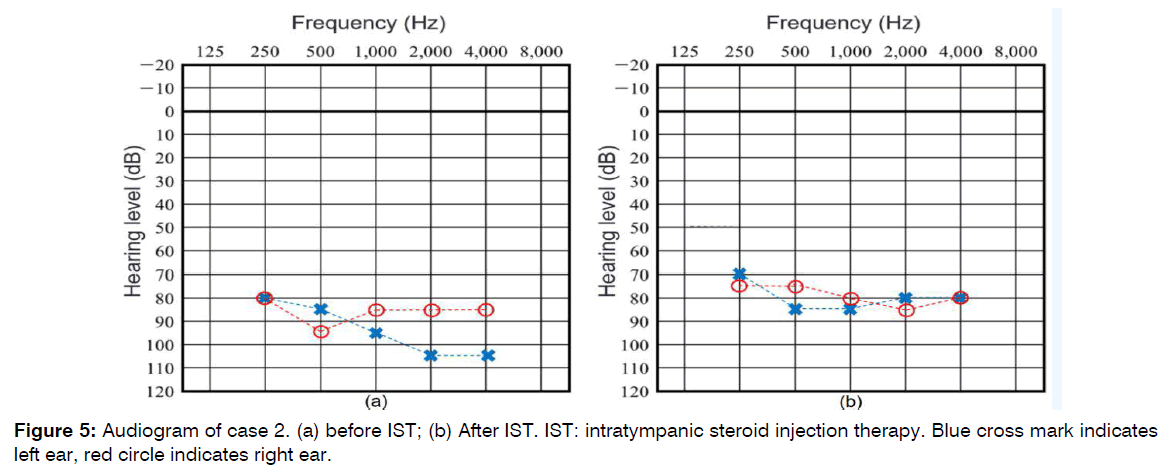
Figure 5: Audiogram of case 2. (a) before IST; (b) After IST. IST: intratympanic steroid injection therapy. Blue cross mark indicates left ear, red circle indicates right ear.
Discussion
Factors in Late-Onset Hearing Loss Caused by CMV: Many uncertainties still remain about impaired hearing and inflammation due to CMV infection. Loss of myosin expression in outer hair cells, generation of active oxygen due to accumulation of activated macrophages, and disruption of hearing-related genes due to chromosomal damage have been thought to be caused by CMV infection [10-12]. but remain to be elucidated. The factors behind late-onset hearing loss caused by cCMV infection are also still unclear but there may be two factors with different causes behind the same late-onset hearing loss in patients with cCMV infection. The first are viral infections and the second are autoimmune inner ear disorders.
It has been reported that a cross-sectional study found that a low viral load in urine specimens obtained during early infancy is associated with a lower risk of hearing loss in patients with cCMV [13]. These are hypotheses related to the former viral factors. However, Ross et al. reported that a study of children with cCMV infection divided into three groups according to age found that in these age groups peripheral blood viral load was not associated with hearing loss [14]. The possibility that hearing loss is triggered by something other than the virus had already been suggested and, in the cases reported here, tests carried out on the patients who came to the hospital due to hearing loss failed to detect CMV in either their blood or their urine. We didn’t know whether there was active virus in the inner ear. However, in a report of mice with SNHL induced by intraventricular inoculation of MSMV, Ikuta et al. reported that the viral antigen and DNA were detected in the spiral ganglion, around the meninges, and in the perilymphatic region at one week after infection, but were lost at two weeks or late [15]. We speculate that there was no CMV in the inner ear either.
We believe that this may be because something such as the entry of another virus triggered the abnormal production of the antibodies which were produced when the patients were first infected with CMV and these antibodies initiated autoimmunity, causing inflammation in the inner ear and in turn hearing loss. In other words, this is a hypothesis related to the latter autoimmune factors. Given also that a linkage between CMV and arteriosclerosis and Autoimmune Diseases (AID) has also been suggested [16], this possibility is conceivable.
In our case reports, there were tendencies for the improvement by IST to be larger in the high register than in the low register. In case 1, Frequency 8000 Hz of left ear changed from 50 to 5. It was the best improvement. And in case 2, Frequency 4000 Hz and 2000 Hz of left ear changed from 105 to 80. These were the best improvement. We attributed this to the induction of inflammation that promote premature aging. And this inflammation might be due to the cross-reactive autoimmune inner ear disorders. Halenius et al. reported the review of the possibility of relationship between human cytomegalovirus (HCMV) and AID by considering following reports [17].
IL-6 and TNFα are also inflammatory cytokines that promote premature aging [18,19]. Induction of IL-6 and TNF�� has been described in HCMV-positive persons [20].
To summarize as a hypothesis, if a patient has a congenital cytomegalovirus infection and have hearing loss immediately after birth, we consider it to be a hearing loss due to inflammation caused by cytomegalovirus. In the case of late onset as in these cases which we reported, it might be deafness due to cross-immunity. In other words, in the case of late onset, there is no virus in the urine after a long period of time. Due to colds and allergies etc., inflammation occurs in the inner ear and deafness progresses.
Intratympanic Steroid Injection: IST is performed under a microscope. In case 1, since a blood sample had also been collected without a problem, the consent of the guardian and the patient was obtained to use a 1 ml syringe with 29G needle for the endoscopically-assisted administration of 0.4 ml dexamethasone to the posterior superior quadrant through the tympanic membrane, avoiding the ossicles, without anesthesia. In case 2, the same procedure was carried out with iontophoretic anesthesia. Iontophoretic anesthesia is a method in which an anesthetic solution is placed in the ear canal and a small amount of electric current is applied to the eardrum so that anesthesia is often applied. When operating on young children, we can’t apply anesthesia. Children are fixed in place with restraint so as not to move. There is still no established therapy for SNHL caused by cCMV. There are reports of systemic administration of anti-CMV drugs for the treatment of congenital hearing loss in cCMV. However, late-onset hearing loss in patients with cCMV infection is surely attributable to different factors and requires different treatment depending on whether CMV is detected or not, as in the cases reported here. Some physicians may administer anti-viral drugs aware of the risk of adverse effects while some physicians treating patients with severe hearing loss and with no other symptoms after stabilization of hearing may adopt a “watch and wait” approach, avoiding the risk of side effects of anti-viral drugs.
There have been some previous reports that intravenous administration of the antiviral agent ganciclovir and oral administration of the antiviral agent valganciclovir are effective in improving SNHL or preventing its progression in children who have developed cCMV infection and been diagnosed with SNHL [21-23]. In the two cases reported here, since CMV was not detected, anti-viral drugs were, not considered as an option and IST was given instead. This may offer a new therapeutic option for the future.
IST has few systemic side effects [24]. In addition, the synthetic glucocorticoid dexamethasone is reported to achieve much higher (4.4- to 189.6-fold) local concentrations in the inner ear with intratympanic injection than with intravenous infusion [25-27]. IST has huge benefits because it has few side effects and enables delivery of a higher concentration of drug.
Oral steroid therapy or intravenous steroid infusion is generally used for the treatment of sudden deafness or progressive hearing loss. These treatments are generally for adults but rare for children. Because the glucocorticoid receptor is present in the inner ear [28-30], and dexamethasone, which has high affinity for glucocorticoid, has been found to reduce intracochlear inflammation [31], steroids have been used for the treatment of sudden deafness attributable to various causes.
Intratympanic steroids have received attention because transport of high concentrations of steroids to the inner ear is expected to bring about higher efficacy, but drug transport is inhibited by the blood-labyrinth barrier with oral therapy or intravenous infusion [32].
Steroids have been reported to increase microvascular blood flow in the cochlea [33], and this increase in blood flow is thought to be due to activated potassium ions associated with angiogenesis in the stria vascularis [34].
Possibility that IST is Effective before Hearing Stabilizes: In patients with hearing loss in one ear, it is easy to notice hearing loss in the other ear. However, in patients who can hear with both ears originally, it is more difficult to notice the severity of an abnormality despite hearing loss in one ear. Similarly in the case discussed here, when the patient visited our hospital at age 6, it was already 3 weeks since the mother sensed a deterioration in the patient’s hearing. At that time, no improvement was observed even after IST, partly due to the time that had elapsed prior to the consultation.
However, at the time of onset at age 8, the patient was examined 2 days after onset and hearing improved as a result of IST.
Patients may become more aware of their symptoms as they grow older but since patients with cCMV usually suffer rapidly progressive hearing loss caused by inflammation whilst they are very young, regular visits to hospital to check whether there is anything wrong with their hearing are probably advisable. It is also important to raise awareness among guardians. It has also been reported that in 75% of cases of late-onset hearing loss among cCMV patients, onset occurs during the first 24 months of life.
Patient Perspective: Case 1 is not only the children but also their parents were worried about the progress of deafness. They were even more worried because the treatment was delayed and they were told that there was no effective treatment. However, with this treatment we were able to stop the progress and had great hope for the future. Case 2 is almost the same as Case 1. It was said at some ENT of University that only cochlear implants could be treated, but this treatment was able to prevent it. Being able to live with hearing aids was a sufficient effect.
Conclusion
It was suggested that IST may be effective for treating cCMV patients presenting with hearing loss when CMV is not detected. The factors behind development of hearing loss when CMV is not detected are unclear but it is possible that something triggered the abnormal production of the antibodies produced when patients were first infected with CMV and these antibodies initiated autoimmunity, causing inflammation and in turn hearing loss. However, this is a hypothesis and the evidence is inadequate. To confirm an autoimmune disease by blood test may help the case like we reported. We hope you will bear in mind that late-onset hearing loss in patients with cCMV may be caused by other factors besides CMV. IST is not major method now, but we hope that further data which gathered on this treatment will establish evidence of it.
Conflict of Interest
The authors declare no conflict of interest. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Written informed consent has been obtained from the patients to publish this paper.
References
- Fletcher KT, Horrell EM, Ayugi J, Irungu C, Muthoka M, Creel LM, et al. The natural history and rehabilitative outcomes of hearing loss in congenital cytomegalovirus: a systematic review. Otol & Neurotol: official publication of the American Otological Society, Am Neurotol Soc [and] Eur Acad of Otol and Neurotol. 2018;39(7):854.
- Dreher AM, Arora N, Fowler KB, Novak Z, Britt WJ, Boppana SB, et al. Spectrum of disease and outcome in children with symptomatic congenital cytomegalovirus infection. The J Pediatr. 2014;164(4):855-9.
- Eichhorn SK. Congenital cytomegalovirus infection: a significant cause of deafness and mental deficiency. Am Ann of the Deaf. 1982;127(7):838-43.
- Yamaguchi A, Oh-Ishi T, Arai T, Sakata H, Adachi N, Asanuma S, et al. Screening for seemingly healthy newborns with congenital cytomegalovirus infection by quantitative real-time polymerase chain reaction using newborn urine: an observational study. BMJ Open. 2017;7(1):e013810.
- Fowler KB, McCollister FP, Dahle AJ, Boppana S, Britt WJ, Pass RF. Progressive and fluctuating sensorineural hearing loss in children with asymptomatic congenital cytomegalovirus infection. The J Pediatr. 1997;130(4):624-30.
- Williamson WD, Demmler GJ, Percy AK, Catlin FI. Progressive hearing loss in infants with asymptomatic congenital cytomegalovirus infection. Pediatr. 1992;90(6):862-6.
- Sakata E, Nakazawa H, Iwashita N. Therapy of tinnitus. Tympanic cavity infusion of lidocaine and steroid solution. Auris Nasus Larynx. 1984;11(1):11-8. German.
- Sakata E, Itoh A, Itoh Y. Treatment of Cochlear-Tinnitus with Dexamethasone Infusion into the Tympanic Cavity. Int Tinnitus J. 1996;2:129-35.
- Sakata H, Suzutani T, Kanzaki S, Ogawa K, Kaga K. Efficacy of transtympanic infusion of dexamethasone into the tympanic cavity in mice with acute sensorineural hearing loss associated with cytomegalovirus infection. Acta Oto-Laryngol. 2022:1-6.
- Pecha PP, Almishaal AA, Mathur PD, Hillas E, Johnson T, Price MS, et al. Role of Free Radical Formation in Murine Cytomegalovirus-Induced Hearing Loss. Otolaryngol Head Neck Surg. 2020;162(5):709-17.
- Ikuta K, Ogawa H, Hashimoto H, Okano W, Tani A, Sato E, et al. Restricted infection of murine cytomegalovirus (MCMV) in neonatal mice with MCMV-induced sensorineural hearing loss. J Clin Virol. 2015;69:138-45.
- Almishaal AA, Mathur PD, Hillas E, Chen L, Zhang A, Yang J, et al. Natural killer cells attenuate cytomegalovirus-induced hearing loss in mice. PLoS Pathogens. 2017;13(8):e1006599.
- Boppana SB, Fowler KB, Pass RF, Rivera LB, Bradford RD, Lakeman FD, et al. Congenital cytomegalovirus infection: association between virus burden in infancy and hearing loss. The J Pediatr. 2005;146(6):817-23.
- Ross SA, Novak Z, Fowler KB, Arora N, Britt WJ, Boppana SB. Cytomegalovirus blood viral load and hearing loss in young children with congenital infection. The Pediatr Inf Dis J. 2009;28(7):588.
- Pass RF. Congenital cytomegalovirus infection and hearing loss. Herpes: The J of the IHMF. 2005;12(2):50-5.
- Söderberg-Nauclér C. Does cytomegalovirus play a causative role in the development of various inflammatory diseases and cancer?. J Int Med. 2006 ;259(3):219-46.
- Li CW, Yu K, Shyh-Chang NG, Li GX, Jiang LJ, Yu SL, et al. Circulating factors associated with sarcopenia during ageing and after intensive lifestyle intervention. J Cachexia, Sarcopenia and Muscle. 2019;10(3):586-600.
- Milan-Mattos JC, Anibal FD, Perseguini NM, Minatel V, Rehder-Santos P, Castro CA, et al. Effects of natural aging and gender on pro-inflammatory markers. Braz J Med and Biol Res. 2019;52.
- Pawelec G, McElhaney JE, Aiello AE, Derhovanessian E. The impact of CMV infection on survival in older humans. Current Opinion in Immunol. 2012;24(4):507-11.
- Kimberlin DW, Lin CY, Sánchez PJ, Demmler GJ, Dankner W, Shelton M, et al. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. The J Pediatr. 2003;143(1):16-25.
- Kimberlin DW, Jester PM, Sánchez PJ, Ahmed A, Arav-Boger R, Michaels MG, et al. Valganciclovir for symptomatic congenital cytomegalovirus disease. New Eng J Med. 2015;372(10):933-43.
- Pasternak Y, Ziv L, Attias J, Amir J, Bilavsky E. Valganciclovir is beneficial in children with congenital cytomegalovirus and isolated hearing loss. The J of Pediatr. 2018;199:166-70.
- Zhao D, Tong B, Wang Q, Hellstrom S, Duan M. A comparison of effects of systemic and intratympanic steroid therapies for sudden sensorineural hearing loss: A meta-analysis. J Otol. 2016;11(1):18-23.
- Sato H, Tabayashi N, Yagi N, Honjo I. Uptake of Steroid in the Inner Ear by Intratympanic Injection of CMC Steroid. Ear Res Japan. 1988;19(1):34-6.
- Parnes LS, Sun AH, Freeman DJ. Corticosteroid pharmacokinetics in the inner ear fluids: an animal study followed by clinical application. The Laryngoscope. 1999 ;109(S91):1-7.
- Chandrasekhar SS, Rubinstein RY, Kwartler JA, Gatz M, Connelly PE, Huang E, et al. Dexamethasone pharmacokinetics in the inner ear: comparison of route of administration and use of facilitating agents. Otolaryngol—Head and Neck Surg. 2000;122(4):521-8.
- Rarey KE, Luttge WG. Presence of type I and type II/IB receptors for adrenocorticosteroid hormones in the inner ear. Hear Res. 1989;41(2-3):217-21.
- Terakado M, Kumagami H, Takahashi H. Distribution of glucocorticoid receptors and 11 beta-hydroxysteroid dehydrogenase isoforms in the rat inner ear. HearResh. 2011;280(1-2):148-56.
- Shimazaki T, Ichimiya I, Suzuki M, Mogi G. Localization of glucocorticoid receptors in the murine inner ear. The Ann Otology, Rhinol & Laryngol. 2002;111(12):1133.
- Lyu AR, Kim DH, Lee SH, Shin DS, Shin SA, Park YH. Effects of dexamethasone on intracochlear inflammation and residual hearing after cochleostomy: A comparison of administration routes. PLoS One. 2018;13(3):e0195230.
- Nyberg S, Abbott NJ, Shi X, Steyger PS, Dabdoub A. Delivery of therapeutics to the inner ear: The challenge of the blood-labyrinth barrier. Sci Trans Med. 2019;11(482):eaao0935.
- Shirwany NA, Seidman MD, Tang W. Effect of transtympanic injection of steroids on cochlear blood flow, auditory sensitivity, and histology in the guinea pig. The Am J Otol. 1998;19(2):230-5.
- Umaru B, Pyriochou A, Kotsikoris V, Papapetropoulos A, Topouzis S. ATP-sensitive potassium channel activation induces angiogenesis in vitro and in vivo. J Pharmacol and Exp Ther. 2015;354(1):79-87.
- Goderis J, Keymeulen A, Smets K, Van Hoecke H, De Leenheer E, Boudewyns A, et al. Hearing in children with congenital cytomegalovirus infection: results of a longitudinal study. The J of Pediatr. 2016;172:110-5.
1Division of Otorhinolaryngology, Kawagoe Ear Institute, Kawagoe Mine Medical Center, Kawagoe City, Saitama, Japan.
2Division of Infectious Diseases and Immunology, Allergy, Saitama Children’s Medical Center, 1-2 Shin-toshin, Chuo-ku Saitama City, Saitama, 330-8777, Japan.
Send correspondence to:
Hideaki Sakata M.D., Ph.D.
Division of Otorhinolaryngology, Kawagoe Ear Institute, Kawagoe Mine Medical Center, 103 Wakitamachi, Kawagoe City, Saitama, 350-1122, Japan, Tel No: +81 49 226 3387, Email: sakata@jikagaku.jp
Paper submitted on October 25, 2022; and Accepted on November 14, 2022
Citation: Hideaki Sakata, Ken Hayashi, Eisuke Suganuma, Tsutomu Oishi. Case Reports on Effect of Intratympanic Steroid Injection Therapy on Late-Onset Hearing Loss in Congenital Cytomegalovirus Infection. Int Tinnitus J. 2022;26(2):115-121.


