The International Tinnitus Journal
Official Journal of the Neurootological and Equilibriometric Society
Official Journal of the Brazil Federal District Otorhinolaryngologist Society
ISSN: 0946-5448

Google scholar citation report
Citations : 12717
The International Tinnitus Journal received 12717 citations as per google scholar report
The International Tinnitus Journal peer review process verified at publons
Indexed In
- Excerpta Medica
- Scimago
- SCOPUS
- Publons
- EMBASE
- Google Scholar
- Euro Pub
- CAS Source Index (CASSI)
- Index Medicus
- Medline
- PubMed
- UGC
- EBSCO
Volume 27, Issue 2 / December 2023
Research Article Pages:217-224
10.5935/0946-5448.20230033
Impact Of Sensorineural Hearing Loss On Subjective Tinnitus Quality In Patients With Bilateral Tinnitus
Authors: Mirta Pe?ek*, Domagoj ?ari??, Zrinka ??usti??, An??ela Margan Nikoli??, Golda Grinblat, Andro Ko?ec
PDF
Abstract
Introduction: Tinnitus is a frequent condition that indicates the sensation of sound in the absence of a corresponding external stimulus and can significantly impair the quality of life. The main risk factor for developing tinnitus is hearing loss. The diagnosis of tinnitus is based on history, assessment of tinnitus severity, clinical examination, and audiological tests. The main purpose of this research was to examine the relationship between the presence and level of hearing loss and the characteristics of tinnitus in patients with bilateral subjective tinnitus. Methods: Total number of 50 participants, 20 men, and 30 women were included in the research. Demographic data, data on hearing impairment obtained by tone audiometry, and data on difficulties caused by tinnitus obtained in two questionnaires - Tinnitus Handicap Inventory (THI) and Tinnitus Functional Index (TFI) were used. Results: Age above 30 years is significantly associated with tinnitus with hearing loss. Hearing impairment is also significantly more often associated with an auditory TFI index >6.7, a total THI index >20, and an emotional THI index >3. Hearing loss was noted in 76% of patients. Conclusion: Tinnitus represents a significant burden for patients, therefore it is important to assess the impact of tinnitus on daily activities and quality of life.
Keywords: Tinnitus; sensorineural hearing loss; Normal hearing; Auditory processing, Subjective quality.
Introduction
Tinnitus can be defined as a sensation of sound in the absence of a corresponding external stimulus. It is a frequent but pathophysiologically and clinically heterogenous condition, that can significantly reduce patients’ quality of life. Decreased quality of life is usually caused by insomnia, reduced speech discrimination, depression, and reduced ability to focus [1]. Most epidemiological studies estimate tinnitus prevalence between 10 and 15% among adults in Western Europe and the United States [1]. Similar data from African and Asian researchers imply that tinnitus is a significant global burden [2,3]. The prevalence of tinnitus increases with age and it is highest in the age group between 60 and 69 years, where it amounts to 31.4% in the United States [4]. People above the age of 70 are probably less likely to seek medical advice for tinnitus due to their other comorbidities [5]. While some authors report a similar prevalence of tinnitus between genders, tinnitus associated with sensorineural hearing loss is slightly more common in men, which is mostly attributed to increased exposure to industrial noise [6,7]. Sensorineural hearing loss is the main risk factor for the development of tinnitus and almost any pathology of the auditory system can be accompanied by tinnitus [8]. Characteristics of hearing loss affect the character of tinnitus and patients with hearing loss in high frequencies usually describe their tinnitus as a pure tone, while patients with pantonal hearing loss tend to perceive their tinnitus as an irregular noise [9]. A consensus between tinnitus researchers is that tinnitus in patients with hearing loss is a neuroplastic reaction to deafferentation [10]. This concept of tinnitus is analogous to the development of phantom pain after limb amputation [11]. Although some patients with hearing loss never develop tinnitus and some with catastrophic tinnitus have normal tonal audiograms, tinnitus patients without hearing loss tend to have cochlear or outer hair cell lesions [12]. Chronic tinnitus can cause various cognitive dysfunctions but it particularly affects attention and working memory [13,14].
In addition to a detailed history, clinical examination, and audiological and neurotological tests, estimation of tinnitus severity is a crucial part of the diagnostic protocol for tinnitus. This is usually performed using standardized questionnaires, such as Tinnitus Handicap Inventory (THI) and Tinnitus Functional Index (TFI) [15]. Both questionnaires estimate the severity of tinnitus well but TFI is superior for evaluating patients’ response to therapy [16]. Also, results of TFI obtained from different linguistic areas may provide valuable conclusions about the impact of tinnitus on patients from various socioeconomic and cultural backgrounds [17].
While the body of knowledge about the association between hearing status and severity of tinnitus has grown during recent years, more data on the impact of hearing loss on tinnitus characteristics is still needed [17]. The overall aim of this study was to examine the relationship between the presence and level of hearing loss with the characteristics of tinnitus in patients with bilateral subjective tinnitus. The specific aims of the study were to determine the characteristics of tinnitus in patients with hearing impairment and those with normal hearing considering their age, sex, degree of hearing loss and involvement of specific frequencies, total score and subscores of the TFI, and total score and subscores of the THI.
Patients and Method
This was a cross-sectional observational study conducted at a tertiary referral center for hearing and balance disorders. All data was collected in accordance with regulations regarding the protection of personal data and with written consent from the patients. The research has been approved by the competent ethical board according to the current criteria of good clinical practice and the Helsinki Declaration.
The study included 50 patients of both sexes with bilateral subjective tinnitus with or without bilateral symmetrical sensorineural hearing loss who were examined in between September 2022 and February 2023. Exclusion criteria were: disease associated with tinnitus (i.e. Ménière’s disese), unilateral tinnitus, unilateral asymmetrical hearing loss, and incomplete data. Patients’ demographic data, Pure Tone Audiometry (PTA) findings, and data on disability caused by tinnitus acquired using Croatian versions of the TFI and THI were used. The THI consists of 25 questions to which participants can respond with „Yes“, „Sometimes“ and „No“. The responds are scored with four, two or zero points, resulting in a total score ranging from 0 to 100. Considering the results of the THI, participants’ tinnitus can be classified into five groups: weak (0-16), medium (18-36), moderate (38-56), severe (58-76), and catastrophic (78-100) [18].The TFI consists of 25 questions to which participants respond with a value between 1 and 10. According to the results of the TFI, participants can be classified into three groups based on their scores: mild annoyance (<25), moderate annoyance (25-50), and severe annoyance (>50) [19].
The relevant anamnestic data (participants’ age and gender), PTA results and scores of TFI and THI questionnaires were stored in a Microsoft Office Excel file. The data were encoded to ensure the anonymity of the participants.
The data were statistically analyzed using the SPSS software (Version 22.0. Released 2013. IBM SPSS Statistics for Windows, Armonk, NY: IBM Corp.). The moment of questionnaire completion was considered as the starting point of the study, and data collection on the level of hearing impairment using PTA was completed on the same day as the final point of the research. The presence and level of hearing impairment were compared with tinnitus characteristics using the analysis of THI nad TFI questionnaires, along with the analysis of subgroups. The dependant variables were the presence of impairment (binary: 0/1) and the presence of scotomas (hearing impairment affecting only one speech frequency) in patients who did not meet the criteria for clinically significant hearing loss (binary: 0/1). The independent variables included age, gender, the overall TFI questionnaire score, and values from the TFI questionnaire subgroups: intrusiveness, sense of control, cognitive impairment, disturbed sleep, auditory phenomena, inability to relax, impact on quality of life, and impact on emotions. Similarly, the same analysis was conducted for overall THI questionnaire score and THI subgroups: functions, emotions, catastrophic tinnitus, and THI disturbance level. The characteristics of the THI and TFI questionnaires were also analyzed according to hearing impairment in dB at individual frequencies from 125 do 8000 Hz and average hearing impairments in dB at overall frequencies, as well as speech frequencies (500-4000 Hz).
The normality of data distribution was checked using the Kolmogorov-Smirnov test, and based on the results, parametric or non-parametric tests were used accordingly, along with appropriate presentation of continuous values (mean and standard deviation or median). A large number of variables were anticipated, so tests that can compensate for unfavorable ratios of participants to variables were employed. In order to mitigate the potential negative impact of a small number of participants on testing a larger number of variables, binary logistic regression was used, which is less sensitive to a large number of variables and allows for the establishment of causal relationships between dependant and tested variables. Subsequently, for each variable showing a statistically significant association with the dependant variable, Receiver Operating Characteristic (ROC) analysis was performed, and the results were presented with the Area Under the ROC Curve (AUC) and the calculated threshold value of maximum sensitivity and specificity using the determination of the Youden J point. The analysis was also verified using the Kruskal-Wallis test for non-binary variables, and significant associations were presented graphically. All statistical tests were two-tailed, and p-values less than or equal to 0.05 were considered statistically significant.
Results
The research involved 50 subjects with bilateral subjective tinnitus. There were 20 male and 30 female participants, which makes a male-female ratio of 2:3. The average age was 52.34 years, with a range of 19 to 80 years of age and a standard deviation of 15.88 years. The average age of men was 50.35 ± 14.92 years, with a median of 47.5 years, and women 53.67 ± 16.35 years, with a median of 57 years.
A statistical analysis of the association between the presence of hearing impairment and the characteristics of tinnitus was performed using a binary logistic regression model, which showed that the presence of hearing impairment as a dependent variable was associated with increasing age of patients (p=0.027, OR 4.9), while the presence of hearing impairment was associated with a higher score of the auditory component of the TFI questionnaire (p=0.001, OR 15.04), a higher score of the THI questionnaire (p=0.037, OR 4.34) and with a higher score of the emotional component of the THI questionnaire (p=0.025, OR 5.04).
These associations were then expressed using ROC curves with associated confidence intervals and sensitivity and specificity values.
Participants older than 30 have significantly more often tinnitus associated with hearing impairment. (AUC 0.719, p=0.023, CI95 0.573-0.866) Youden J cut-off criterion is 30 years, sensitivity 92.1%, specificity 91.7% (Figure 1).
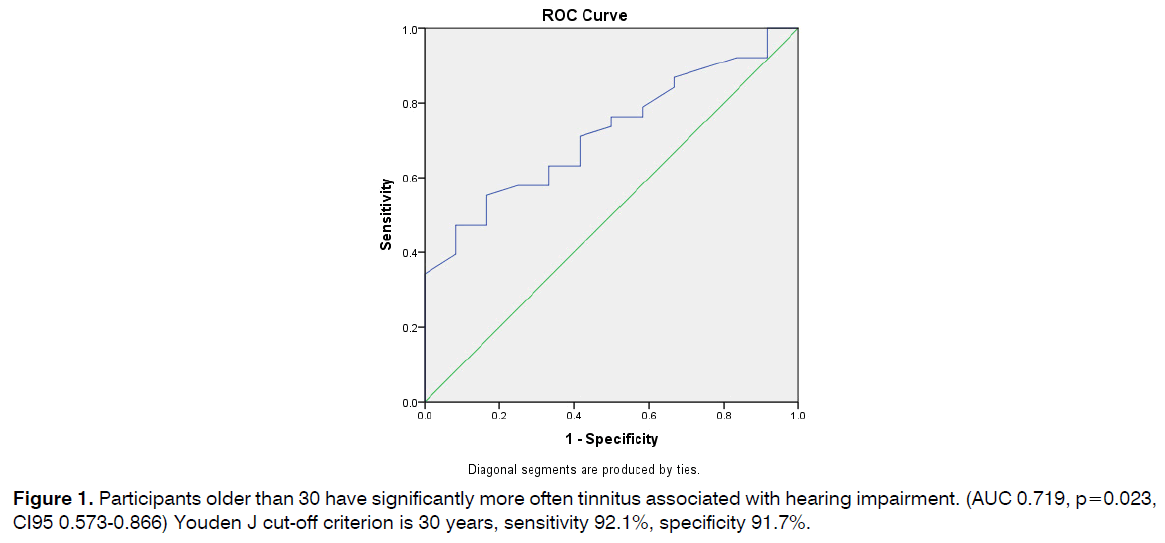
Figure 1: Participants older than 30 have significantly more often tinnitus associated with hearing impairment. (AUC 0.719, p=0.023, CI95 0.573-0.866) Youden J cut-off criterion is 30 years, sensitivity 92.1%, specificity 91.7%.
Participants with hearing impairment have significantly more frequent auditory TFI index >6.7. (AUC 0.866, p=0.001, CI95 0.753-0.980) Youden J cut-off criterion is TFI auditory subgroup, value >6.7, sensitivity 94.7%, specificity 83.3% (Figure 2).
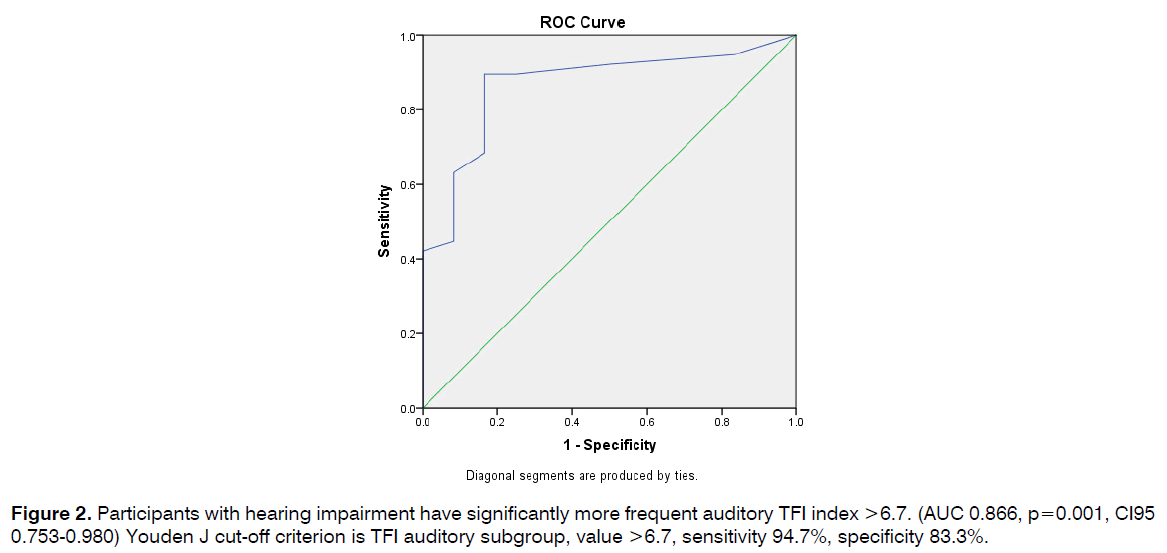
Figure 2: Participants with hearing impairment have significantly more frequent auditory TFI index >6.7. (AUC 0.866, p=0.001, CI95 0.753-0.980) Youden J cut-off criterion is TFI auditory subgroup, value >6.7, sensitivity 94.7%, specificity 83.3%.
Participants with hearing impairment have a significantly more frequent total THI score >20. (AUC 0.698, p=0.040, CI95 0.554-0.843) Youden J cut-off criterion is THI total score, value >20, sensitivity 84.2%, specificity 91.7% (Figure 3).
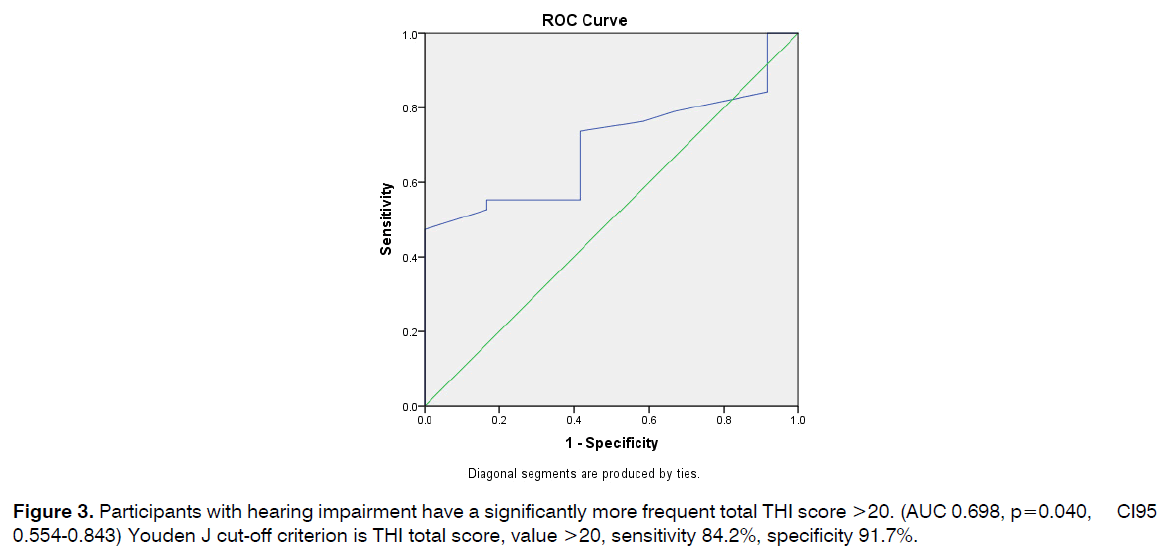
Figure 3: Participants with hearing impairment have a significantly more frequent total THI score >20. (AUC 0.698, p=0.040, CI95 0.554-0.843) Youden J cut-off criterion is THI total score, value >20, sensitivity 84.2%, specificity 91.7%.
Participants with hearing impairment have a significantly more frequent emotional THI score >3. (AUC 0.706, p=0.033, CI95 0.562-0.850) Youden J cut-off criterion is THI emotional subgroup, value >3, sensitivity 94.7%, specificity 91.7% (Figure 4).
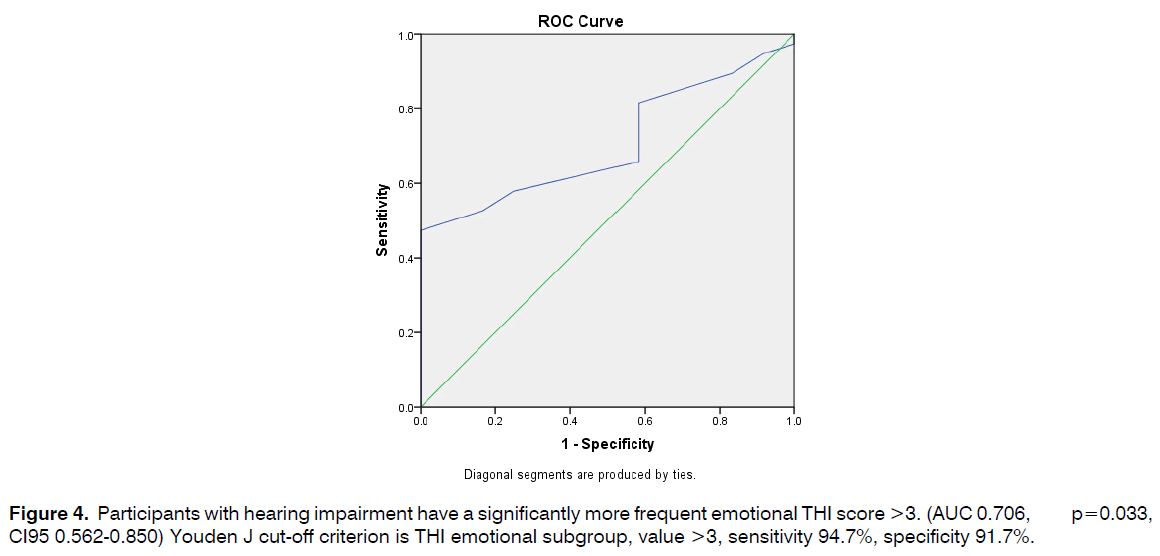
Figure 4: Participants with hearing impairment have a significantly more frequent emotional THI score >3. (AUC 0.706, p=0.033, CI95 0.562-0.850) Youden J cut-off criterion is THI emotional subgroup, value >3, sensitivity 94.7%, specificity 91.7%.
Furthermore, non-parametric Kruskal-Wallis and Mann- Whitney U tests confirmed that age (p=0.023), the auditory subgroup of TFI score (p=0.001), total THI score (p=0.04) and the emotional subgroup of THI score (p=0.032 ) are associated with the presence of hearing impairment. The presence of hearing impairment scotoma in patients who do not meet the criteria for clinically present hearing impairment is associated with higher values of the subgroup of the TFI questionnaire related to sleep difficulties (p=0.044). The average value of the total sum of the TFI questionnaire is 62.07 (range of sum 7.06- 163.53), and THI 40.08 (range of sum 2-90). According to the results of the TFI questionnaire, 7 subjects are mildly disturbed by tinnitus, 9 are significantly disturbed, and 34 are severely disturbed. According to the results of the THI questionnaire, there are 6 very mild difficulties, 18 mild, 15 moderate, 8 serious, and 3 very serious difficulties.
Hearing impairment was recorded in 38 (76%) patients. Among them, 14 (36.84%) are men and 24 (63.16%) are women. Scotomas of hearing loss affecting highfrequency hearing, but not the speech-discriminating frequencies were recorded in 10 patients (20%). Among patients with normal hearing [12], the ratio of males to females is equal, 1:1. The average age of patients with hearing loss is 55.13, and that of patients with normal hearing is 43.83.
The average hearing threshold on the right ear measured by PTA in the range of speech frequencies (500-4000 Hz) is 24.15 dB and on the left ear 28.19 dB. The total average hearing threshold on the right ear measured by PTA is 26.35 dB and on the left ear 30.82 dB. The difference is not statistically significant, considering that 5 dB is taken as the smallest clinically significant difference. The average hearing threshold measured by PTA in the range of speech frequencies for both ears is 26.17 dB, and the total average hearing threshold is 28.59 dB (Table 1).
| Average hearing threshold (dB) | ||
|---|---|---|
| Frequency (kHz) | Left | Right |
| 0.125 | 29.1 | 22.4 |
| 0.25 | 27.3 | 19.1 |
| 0.5 | 24.96 | 17.3 |
| 0.75 | 24.62 | 19.52 |
| 1 | 24.5 | 2.5 |
| 1.5 | 25.26 | 21.98 |
| 2 | 26.4 | 23.2 |
| 3 | 32.6 | 27.4 |
| 4 | 36.9 | 35.6 |
| 6 | 42.7 | 41.4 |
| 8 | 44.7 | 41.5 |
Table 1. The average hearing threshold level as a function of frequency measured by tone audiometry.
Discussion
Tinnitus is a multifactorial symptom, with development induced by hearing loss, physical and psychological diseases, as well as the use of drugs [7]. Only patients in whom no organic cause of tinnitus was identified participated in this study.
According to some studies, tinnitus occurs more often in men, although not all results were statistically significant [7,20-22]. Also, the prevalence of tinnitus in people with preserved hearing is higher in men [5]. In this study, tinnitus was recorded in more women than men (60%: 40%), and the prevalence of tinnitus in patients with preserved hearing is equal in men and women (50%).
Tinnitus is substantially associated with advancing age and most commonly occurs in adults of age 30 to 70 years [7,23]. Tinnitus is frequently associated with progressive hearing loss in adults between the ages of 45 and 55, a condition known as presbytinitus [24]. The results of this research confirm that. In 76% of the patients, temporary hearing loss was recorded, which supports the theory that a reduction in the effective functioning of the efferent cochlear system causes tinnitus [25]. Research conducted by Sanchez et al. And [26,27] Savastano et al. Showed5 that patients with tinnitus who have normal hearing are younger than patients with tinnitus who have a hearing impairment. The same was confirmed by this research (average of 43.83 vs 55.13 years).
The frequency of tinnitus in most studies is similar in groups with and without hearing loss [18]. Patients with hearing loss have higher tinnitus loudness as determined by Minimum Masking Level (MML) testing, but in some studies, the level of tinnitus interference measured by standardized questionnaires such as TFI and Tinnitus Severity Index (TSI) is the same between tinnitus patients with and without hearing loss [5,18]. Other studies report greater subjective tinnitus distress measured by TFI and THI in patients with hearing loss [17]. Although patients with tinnitus and hearing loss in most studies report subjectively greater tinnitus distress (measured, for example, with a visual-analog scale), their tinnitus is also easier to mask, i.e. they experience it less in louder environments and when doing activities [5].
The use of THI and TFI questionnaires is essential in assessing the impairment of quality of life caused by tinnitus [28]. In the category of patients with tinnitus without hearing loss, the prevalence is highest in the age group between 41-50 years [7]. The character of tinnitus in patients without hearing loss depends significantly on the lateralization of noise. Patients with a history of bilateral tinnitus more often report exposure to noise, hypersensitivity to sound, longer duration, and higher frequency of tinnitus [28]. Noise exposure and aging are risk factors for the development of subclinical hearing loss, a condition caused by the loss of synapses between inner sensory cells and auditory nerve fibers with preserved inner and outer sensory cells. This condition is not detected by standard PTA, patients can hear even quiet sounds, but can hardly understand speech in a noisy environment. The loss of auditory nerve synapses leads to a compensatory increase in central neural activity, so tinnitus without hearing loss can be a precursor to diseases that are diagnosed only after the onset of hearing loss [5,29]. Based on auditory evoked potentials, bilateral tinnitus without hearing loss is associated with increased activity of the cochlear nuclei. Research shows increased amplitudes and shortened latencies of waves III and V in patients with bilateral tinnitus, and neurons of the ventral cochlear nucleus participate in the generation of these waves [30].
Patients with normal hearing and unilateral tinnitus, on the other hand, do not show severe alterations in objective audiological evaluation, but their findings are more similar to the general population. They also have more tinnitus distress, as measured by the THI questionnaire, than the group with bilateral tinnitus and normal hearing, indicating that higher cortical structures, such as the limbic system and prefrontal cortex, are involved in the development of unilateral tinnitus in people with normal hearing [30]. This pathogenetic mechanism is supported by the fact that there was no discernible relationship between tinnitus intensity and tinnitus distress, neither in those with hearing loss nor in those with normal hearing, and that tinnitus distress is undoubtedly connected to psychological distress states like anxiety and depression [7]. Mahafza et al. in their research proved a more persistent tinnitus determined by TFI, a lower sense of control over tinnitus, a more significant impact on sleep, and greater hearing difficulties in the group of patients with tinnitus and temporary hearing loss compared to patients with tinnitus and normal hearing [17]. Tinnitus in the left ear is more common due to the greater sensitivity of the left cochlea to damage caused by, for example, noise [31].
According to the results obtained in this research, it was shown that age, the auditory subgroup of the TFI score, the total THI score, and the emotional subgroup of the THI score are related to the presence of hearing impairment.
The association of the auditory subgroup of TFI results and acquired hearing impairment was also confirmed in the research conducted by Mahafza et al., which indicates that individuals with acquired hearing loss and tinnitus have more difficulties related to hearing [17]. Therefore, the auditory subgroup of the TFI questionnaire may provide useful information to identify the characteristics of different hearing-related difficulties in tinnitus patients, which influence the severity of tinnitus-related suffering. According to research conducted by Fackrell et al. [32,33], the auditory subgroup of the TFI questionnaire did not contribute to the overall concept of assessing the functional effect of tinnitus in their research. Moreover, they suggest that the TFI questionnaire be modified and that the auditory subgroup be omitted from it [32,33].
Mahafza et al. proved that the state of hearing has an impact on the following subgroups: intrusiveness, sense of control, sleep, listening quality, ability to relax, and quality of life. However, no difference was found between the cognitive and emotional subgroups in patients with and without hearing impairment. One possible explanation is that both cognitive and emotional subgroups reflect a general aspect of tinnitus severity that does not depend on hearing status.
To the best of our knowledge, the connection between the emotional subgroup of the THI questionnaire and sensorineural hearing loss has not been described in the literature so far, so this result represents a new contribution to the understanding of the effect of tinnitus and sensorineural hearing loss on the emotional state of the patient.
The research conducted by Martines et al.7showed that 23.1% of patients, according to the results of the THI questionnaire, have difficulties with sleeping and performing daily activities. Also, in the same research, it was assumed that the discomfort associated with the presence of tinnitus, especially in people with normal hearing, is caused by a certain degree of psychological distress and attention that interfere with habituation. The authors also state that the patient’s reaction to tinnitus cannot be classified as a simple function of its psychoacoustic aspects, but also as a complex interaction between acoustic phantom symptoms, attention and symptoms of depression [7]. In our study, 37 (74%) patients with tinnitus have difficulty sleeping, which is related to the presence of hearing impairment scotoma.
Most patients with tinnitus seek specialist help when tinnitus starts to interfere with their daily activities. The feeling of discomfort caused by the presence of tinnitus occurs more often in patients with hearing loss. This may indicate that hearing loss significantly increases tinnitus distress, even if the hearing loss is not severe. However, the THI results from the research conducted by Savastano show that tinnitus, even if it is not accompanied by hearing loss, can cause sleep disturbances and difficulties in performing daily activities. On the other hand, when tinnitus is associated with hearing loss, it can only be heard in a quiet environment, easily masked and forgotten during activities [5].
Dauman et al. stated in their work that the shared experiences of patients with tinnitus enabled a better understanding of their condition because their experiences and perceptions in different circumstances were taken into account. Patients with chronic tinnitus stated that they are extremely frustrated because they cannot control the occurrence of tinnitus. They suggest individualized counseling and psychotherapeutic approaches should be aimed at reducing the mentioned frustrations [34].
The limitations of this study are a small sample, a cross-sectional study, and the use of only PTA in the assessment of hearing impairment, as the use of additional electrophysiological methods could complement the information obtained by subjective methods of tinnitus assessment. However, since tinnitus is a purely subjective category, and this research focused on the burden of the disease and quality of life, we believe that the collected data are sufficient for an insight into the connection between hearing and the quality of tinnitus, which has not been investigated so far.
Conclusion
Tinnitus, in addition to representing a significant global burden, affects the daily activities and quality of life of the patient. Associations between the emotional THI questionnaire subgroup and presence of sensorineural hearing loss helps us to understand the impact of tinnitus and chronic hearing loss on the patient’s emotional state. Therefore, the use of THI and TFI questionnaires plays an important role in assessing the specific emotional impact of tinnitus on the patient’s daily life.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Acknowledgements
None to declare.
References
- Tunkel DE, Bauer CA, Sun GH, Rosenfeld RM, Chandrasekhar SS, Cunningham Jr ER, et al. Clinical practice guideline: tinnitus. Otolaryngol Head Neck Surg. 2014;151:S1-40.
- Xu X, Bu X, Zhou L, Xing G, Liu C, Wang D. An epidemiologic study of tinnitus in a population in Jiangsu Province, China. J Am Acad Audiol. 2011;22(09):578-85.
- Lasisi AO, Abiona T, Gureje O. Tinnitus in the elderly: Profile, correlates, and impact in the Nigerian Study of Ageing. Otolaryngol Head Neck Surg. 2010;143(4):510-5.
- Shargorodsky J, Curhan GC, Farwell WR. Prevalence and characteristics of tinnitus among US adults. Am J Med. 2010;123(8):711-8.
- Savastano M. Tinnitus with or without hearing loss: are its characteristics different?. Eur Arch Oto-Rhino. 2008;265:1295-300.
- Jarach CM, Lugo A, Scala M, van den Brandt PA, Cederroth CR, Odone A, et al. Global prevalence and incidence of tinnitus: A systematic review and meta-analysis. JAMA Neurol. 2022.
- Martines F, Bentivegna D, Martines E, Sciacca V, Martinciglio G. Assessing audiological, pathophysiological and psychological variables in tinnitus patients with or without hearing loss. Eur Arch Oto-Rhino. 2010;267:1685-93.
- Esmaili AA, Renton J. A review of tinnitus. Aust J Gen Pract. 2018;47(4):205-8.
- Langguth B, Landgrebe M, Schlee W, Schecklmann M, Vielsmeier V, Steffens T, et al. Different patterns of hearing loss among tinnitus patients: a latent class analysis of a large sample. Front Neurol. 2017;8:46.
- Eggermont JJ, Roberts LE. The neuroscience of tinnitus.Trends Neurosci. 2004;27(11):676-82.
- De Ridder D, Elgoyhen AB, Romo R, Langguth B. Phantom percepts: tinnitus and pain as persisting aversive memory networks. Proc Natl Acad Sci. 2011;108(20):8075-80.
- Baguley D, McFerran D, Hall D. Tinnitus. Lancet. 2013;382(9904):1600-7.
- Tegg-Quinn S, Bennett RJ, Eikelboom RH, Baguley DM. The impact of tinnitus upon cognition in adults: A systematic review. Int J Audiol. 2016;55(10):533-40.
- Mohamad N, Hoare DJ, Hall DA. The consequences of tinnitus and tinnitus severity on cognition: a review of the behavioural evidence. Hear Res. 2016;332:199-209.
- Meikle MB, Henry JA, Griest SE, Stewart BJ, Abrams HB, McArdle R, et al. The tinnitus functional index: development of a new clinical measure for chronic, intrusive tinnitus. Ear hear. 2012;33(2):153-76.
- Fernández M, Cuesta M, Sanz R, Cobo P. Comparison of Tinnitus Handicap Inventory and Tinnitus Functional Index as Treatment Outcomes. Audiol Res. 2023;13(1):23-31.
- Mahafza N, Zhao F, El Refaie A, Chen F. A comparison of the severity of tinnitus in patients with and without hearing loss using the tinnitus functional index (TFI). Int J Audiol. 2021;60(3):220-6.
- Yenigün A, Doğan R, Aksoy F, Akyüz S, Dabak H. Assessment of tinnitus with tinnitus severity index, tinnitus handicap inventory and distortion product otoacoustic emissions in patients with normal hearing and hearing loss. Turk J Ear Nose Throat. 2014;24(1):11-6.
- Henry JA, Griest S, Thielman E, McMillan G, Kaelin C, Carlson KF. Tinnitus Functional Index: Development, validation, outcomes research, and clinical application. Hear Res. 2016;334:58-64.
- Johansson MSK, Arlinger SD. Prevalence of hearing impairment in a population in Sweden. Int J Audiol. 2003;42(1):18–28.
- Palmer KT, Griffin MJ, Syddall HE, Davis A, Pannett B, Coggon D. Occupational exposure to noise and the attributable burden of hearing difficulties in Great Britain. Occup Environ Med. 2002 59(9):634-9.
- Fabijanska A, Rogowski M, Bartnik G, Skarzynski H. Epidemiology of tinnitus and hyperacusis in Poland. In Proceedings of the sixth international tinnitus seminar 1999; 569-571.
- Briner W, House J, O'Leary M. Synthetic prostaglandin E1 misoprostol as a treatment for tinnitus. Arch Otolaryngol Head Neck Surg. 1993;119(6):652-4.
- Fünfgeld EW, Stalleicken D. Objektivierung der klinischen Effekte von Ginkgo-biloba-Extrakt bei cerebraler Insuffizienz mittels Dynamic-Brain-Mapping.1992;117-128. Springer Berlin Heidelberg.
- Eggermont JJ. Central tinnitus. Auris Nasus Larynx. 2003;30:7-12.
- Sanchez TG, Ferrari GM. O controle do zumbido por meio da prótese auditiva: sugestões para otimização do uso. Pró-fono. 2002:111-8.
- Sanchez TG, Bento RF, Miniti A, Camara J. Zumbido: características e epidemiologia: experência do Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo. Braz J Otorhinolaryngol. 1997;63(3):229-35.
- Zagólski O, Stręk P. Comparison of characteristics observed in tinnitus patients with unilateral vs bilateral symptoms, with both normal hearing threshold and distortion-product otoacoustic emissions. Acta Otolaryngol. 2017;137(2):174-8.
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29(45):14077-85.
- Song K, Shin SA, Chang DS, Lee HY. Audiometric profiles in patients with normal hearing and bilateral or unilateral tinnitus. Otol Neurotol. 2018;39(6):e416-21.
- Nageris BI, Raveh E, Zilberberg M, Attias J. Asymmetry in noise-induced hearing loss: relevance of acoustic reflex and left or right handedness. Otol Neurotol. 2007;28(4):434-7.
- Fackrell K, Hall DA, Barry JG, Hoare DJ. Psychometric properties of the Tinnitus Functional Index (TFI): Assessment in a UK research volunteer population. Hear Res. 2016;335:220-35.
- Fackrell K, Hall DA, Barry JG, Hoare DJ. Performance of the Tinnitus Functional Index as a diagnostic instrument in a UK clinical population. Hear Res. 2018;358:74-85.
- Dauman N, Erlandsson SI, Albarracin D, Dauman R. Exploring tinnitus-induced disablement by persistent frustration in aging individuals: A grounded theory study. Front aging neurosci. 2017;9:272.
1School of Medicine, University of Zagreb, Šalata 3b, Zagreb, Croatia
2Department of Otorhinolaryngology and Head and Neck Surgery, University Hospital Center Sestre milosrdnice, Zagreb, Croatia
3SUVAG Polyclinic Zagreb, Croatia
4Bruce Rappaport Faculty of Medicine, Technion-Israel Institute of Technology, Haifa, Israel
5Department of Otorhinolaryngology, Hillel Yaffe Medical Center, Hadera, Israel
Send correspondence to:
Mirta Peček
School of Medicine, University of Zagreb, Šalata 3b, Zagreb, Croatia,
E-mail: mirta.pec@gmail.com
Tel: +385912312303
Paper submitted on November 12, 2023; and Accepted on November 30, 2023
Citation: Peček M. Impact Of Sensorineural Hearing Loss On Subjective Tinnitus Quality In Patients With Bilateral Tinnitus. Int Tinnitus J. 2023;27(2):217-224.


