The International Tinnitus Journal
Official Journal of the Neurootological and Equilibriometric Society
Official Journal of the Brazil Federal District Otorhinolaryngologist Society
ISSN: 0946-5448

Google scholar citation report
Citations : 12717
The International Tinnitus Journal received 12717 citations as per google scholar report
The International Tinnitus Journal peer review process verified at publons
Indexed In
- Excerpta Medica
- Scimago
- SCOPUS
- Publons
- EMBASE
- Google Scholar
- Euro Pub
- CAS Source Index (CASSI)
- Index Medicus
- Medline
- PubMed
- UGC
- EBSCO
Volume 27, Issue 2 / December 2023
Research Article Pages:183-190
10.5935/0946-5448.20230028
Investigation of IgG Titers in Hemodialysis Patients and Controls Following Administration of the COVID-19 Vaccine
Authors: Manizheh Jozpanahi, Mohammad Moharrer, Pouria Mahmoudi, Seyedeh Pegah Saeed, Hossein Dinmohammadi*
PDF
Abstract
Introduction: End-Stage Renal Disease (ESRD) patients necessitate dialysis when kidney transplantation is not feasible. Hemodialysis patients exhibit higher mortality rates compared to the general population due to uremia and an increased burden of comorbidities. In this vulnerable population, defective innate and adaptive immunity contribute to infectious diseases being a leading cause of hospitalization and mortality. Given the COVID-19 pandemic and the potential for a diminished antibody response to the COVID-19 vaccine among dialysis patients, this study aimed to measure IgG antibody titers in this patient group following COVID-19 vaccination and compare them to healthy individuals. Materials and Methods: This cross-sectional study enrolled hemodialysis patients who had received a minimum of two doses of the COVID-19 vaccine within the past two to six months. Informed consent was obtained from the patients for antibody titer testing. Additional information was collected using a checklist. A control group consisting of individuals who had also received at least two doses of the COVID-19 vaccine was selected and matched to the patient group based on vaccine type, number of doses, and timing of administration. Relevant data for both groups were recorded in the checklist. IgG titers were measured using the indirect ELISA technique to quantify specific IgG against the Spike antigen in the serum samples of both patients and controls. A comparison of IgG titers between the two groups was conducted using SPSS version 26 software. Results: The hemodialysis group comprised 44.1% males, while the control group consisted of 67.6% males. The mean age for the hemodialysis and control groups was 59.97±15.92 and 54.79±21.77, respectively. Underlying diseases were present in 76.5% of the hemodialysis group and 58.8% of the control group, with hypertension being the most common comorbidity in both groups. Sinopharm was the most commonly administered vaccine in both groups for both the first and second doses. Vaccine side effects were reported by 50% of hemodialysis patients and 17.6% of the control group. Furthermore, 55.9% of the hemodialysis group and 35.3% of the control group had a history of prior COVID-19 infection before vaccination. The positive IgG titer rates were 94% in the hemodialysis group and 91% in the control group, with no significant difference observed between the two groups (P<0.999). The relationship between positive IgG titers and group membership was not significant across other investigated variables. Conclusion: The present study revealed no significant difference in IgG titers against the S1 antigen between hemodialysis patients and controls who had received a minimum of two doses of the COVID-19 vaccine. Furthermore, IgG titers were not associated with age, sex, underlying diseases, vaccine side effects, or behavioral parameters. In addition, an inverse correlation was observed between the duration since the last vaccine dose and the initiation of dialysis, and IgG titers.
Keywords: IgG, Hemodialysis, COVID-19.
Introduction
Prevalence of Chronic Kidney Disease (CKD) and End- Stage Renal Disease (ESRD) is increasing worldwide. Many patients progress to ESRD and may require dialysis, even with regular nephrology follow-up [1]. ESRD is a progressive, debilitating, and chronic disease that necessitates nursing and medical interventions [2], with hemodialysis being the primary treatment for patients who are currently or unable to undergo kidney transplantation. The main goal of hemodialysis is to provide adequate and safe treatment to improve the patient’s physical condition and prevent subsequent complications [3].
Considering that patients with ESRD have impaired renal function and disturbances in maintaining homeostasis of waste products, fluids, and electrolytes, dialysis or kidney transplantation becomes crucial in increasing the survival chances of these patients. Hemodialysis is the most common method used to assist patients with ESRD. Due to complications such as uremia and comorbidities including hypertension, diabetes, cardiovascular diseases, and malignancies, patients undergoing hemodialysis have higher mortality rates compared to the general population. Moreover, infectious diseases and sepsis are major causes of hospitalization and mortality in this vulnerable population due to inherent and adaptive immune deficiencies. Additionally, as these patients typically spend a minimum of 10 to 15 hours per week in dialysis facilities in close proximity to other patients and healthcare personnel, this characteristic can increase the risk of transmission of infectious diseases, including COVID-19 [4-6].
Limited information is available on hemodialysis patients with COVID-19 pneumonia [7]. Patients undergoing hemodialysis in relevant centers constitute a unique and vulnerable population throughout the pandemic [8]. In addition to personal hygiene, increased sanitation, social distancing, and isolation, vaccine administration and immunization are vital for managing this highly susceptible population. Hemodialysis patients are at a higher risk of COVID-19 infection due to comorbidities, immunodeficiency, and overcrowding in dialysis centers [9]. Patient age is also an important factor affecting humoral response, regardless of underlying chronic medical conditions [10].
Given the recent COVID-19 pandemic, which has become a widespread infectious disease, it is crucial to prioritize individual hygiene, enhanced cleanliness, social distancing, isolation, vaccine production, and immunization for this vulnerable population. Hemodialysis patients may have a lower immune response to COVID-19 vaccination compared to the general population. Therefore, in this study, we aimed to measure the IgG antibody titer in hemodialysis patients who have also received the COVID-19 vaccine.
Materials and Methods
Design
This cross-sectional study aimed to investigate hemodialysis patients and non-dialysis patients who received the Covid-19 vaccine in the dialysis centers and hospitals of Zanjan city in 2022. Sampling was conducted on eligible patients who met the inclusion and exclusion criteria. Inclusion criteria consisted of hemodialysis patients and control subjects who had received at least two doses of the Covid-19 vaccine. Exclusion criteria included hemodialysis patients or control subjects who did not receive the Covid-19 vaccine for any reason, individuals who had not completed a minimum of 2 weeks since their second or third vaccine dose, and patients or controls under the age of 14.
Sample size calculation was based on a study by Grupper et al. [11] and the average comparison formula in two populations. The sample size for each group was determined to be 34 individuals using the following formula and given values: S1=3350/37, S_2=89/8728, d=4501/00, α=0.05, and β=0.80. Thus, a total of 68 people were included in the study, with 34 being hemodialysis patients and 34 being healthy individuals who had received at least two doses of the Covid-19 vaccine.
Procedure
The procedure began with obtaining confirmation and ethical approval. Venous blood samples were collected from hemodialysis patients attending the dialysis center and sent to the laboratory. A checklist was completed for each participant via telephone interview, covering information such as age, gender, BMI, comorbidities, behavioral parameters, vaccine details, history of previous Covid-19 infection, vaccine side effects, and elapsed time since the last dose of the vaccine and the start of dialysis. The Indirect ELISA technique was employed to measure the specific IgG levels against the Spike antigen in the serum of both patient groups.
Data analysis
Data analysis was performed using SPSS software version 26. Descriptive statistics were used to summarize the variables, including mean (standard deviation), median (interquartile range), and frequency (percentage). The two groups were compared using an independent t-test for continuous variables (such as age) and Fisher’s exact test for categorical variables (including underlying diseases, behavioral parameters, vaccine type, side effects, and hospitalization rates). A significance level of 5% was considered.
Ethical declarations
Ethical considerations were taken into account, and the study was conducted after obtaining approval from the Research and Technology Vice-Chancellor of Zanjan University of Medical Sciences (with the code IR.ZUMS. REC.1401.230), acquiring a letter of introduction, and obtaining permission from the director of Valiasr Hospital.
Results
A total of 68 participants were enrolled in the study, with 34 being hemodialysis patients and 34 serving as controls without dialysis. Out of the hemodialysis patients, 15 (44.1%) were male and 19 (55.9%) were female. In the control group, 23 (67.6%) were male and 11 (32.4%) were female. The mean age in the hemodialysis group was 59.97±15.92 years, while in the control group, it was 54.79±21.77 years. There was no significant difference between the two groups in terms of age (P=0.268) and gender (P=0.510). Regarding smoking and alcohol consumption, there was no significant difference between the groups (P=0.200 and P=0.076, respectively). However, the hemodialysis group had significantly less exercise compared to the control group (P=0.029). Specifically, 91.2% of the hemodialysis patients exercised less than 3 days a week, while this percentage was 64.7% in the control group. Additionally, the hemodialysis group had significantly less sleep compared to the control group (P=0.300), with approximately 62% of hemodialysis patients sleeping less than 6 hours compared to 32% in the control group.
Regarding underlying diseases, there was no significant difference between the two groups overall (P=0.120). However, hypertension and hypothyroidism were significantly more prevalent in the hemodialysis group (P=0.006 and P=0.046). Other diseases such as hyperthyroidism, single kidney disease, cancer, liver disease, and kidney disease other than Chronic Kidney Disease (CKD) and End-Stage Renal Disease (ESRD) were observed in a small number of patients in either group. Neither group had patients with a history of chemotherapy, radiotherapy, immunodeficiency, or organ transplantation.
There was no significant difference between the hemodialysis and control groups in terms of the type of COVID-19 vaccine administered (P<0.05). Sinopharm (BBIBP-CorV vaccine) was the most common type of vaccine given in both groups.
Significant differences were observed between the two groups in terms of vaccine side effects (P<0.05), with a higher rate of complications in the hemodialysis group (50% versus 17.6% in the control group). The complications of paresthesia, myalgia, and arthralgia were significantly higher in the hemodialysis group compared to the control group. However, there were no significant differences in other complications such as fever, headache, nausea, diarrhea, and kidney failure, and no cases of respiratory failure, liver failure, heart attack, or stroke were reported in either group.
Comparison of the history of COVID-19 infection before vaccination and hospitalization due to Covid-19 did not reveal any significant differences between the hemodialysis and control groups Table 1.
| Variable | Category | Group | P-value | |
|---|---|---|---|---|
| hemodialysis number (percentage) |
control number (percentage) |
|||
| History of covid-19 infection before the vaccine | has it | 19 (55.9%) | 12 (35.3%) | 0.088 |
| does not have | 15 (44.1%) | 22 (64.7%) | ||
| Hospitalization due to covid-19 | has it | 5 (26.3%) | 2 (16.7%) | 0.676 |
| does not have | 14 (73.7%) | 10 (83.3%) | ||
Table: 1 Comparison of COVID-19 infection before the vaccine and hospitalization due to covid-19.
The positive IgG titer was observed in approximately 94% of the hemodialysis group and 91% of the control group, with no significant difference between the groups (P<0.999). When adjusting for exercise, sleep, and side effects using logistic regression analysis, there was no significant difference in the positive IgG titer between the two groups Table 2.
| IgG titer | Group | No adjustment | adjustment | |||
|---|---|---|---|---|---|---|
| Hemodialysis | control | Test statistics | p-value | p-value | OR (95% confidence interval) |
|
| negative | 2 (5.9%) | 3 (8.8%) | - | 0.999 < | 0.677 | 0.82 ( 45.3 ، 0.11 ) |
| Positive | 32 (94.1%) | 31 (91.2%) | ||||
Table: 2 Comparison of IgG titer in two hemodialysis and control groups.
When investigating the relationship between IgG titer and gender, age (<40 years and >65 years), no significant associations were found.
Furthermore, age was found to have a positive correlation with IgG titer in the hemodialysis group and a negative correlation in the non-hemodialysis group, indicating that IgG titer increases with age in hemodialysis patients but decreases in non-hemodialysis patients Figure 1.
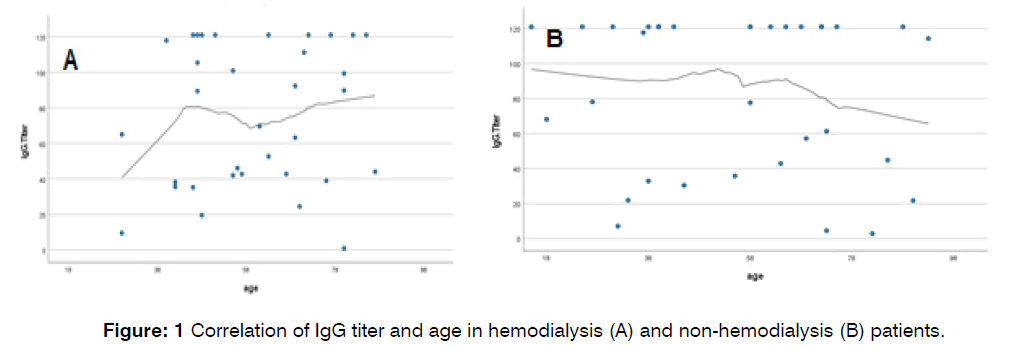
Figure 1: Correlation of IgG titer and age in hemodialysis (A) and non-hemodialysis (B) patients.
The study conducted to compare the levels of IgG titer and various behavioral factors such as exercise, smoking, alcohol consumption, and sleep duration. The results showed no significant relationship was found between exercise frequency (less than 3 days per week: P = 0.295, more than 3 days per week: P = 0.200 P = 0), smoking (P < 0.999), alcohol (P < 0.999), and sleep (more than 6 hours: P < 0.999), and IgG titer. Additionally, the study revealed no significant relationship between IgG titer and underlying diseases, having or not having a certain disease, (having: P = 0.572, not having: P < 0.999), hyperlipidemia (having: P = 0.385, not having: 999 P < 0.00), diabetes mellitus (not present: P = 0.699), and high blood pressure (present: P = 0.513, not present: P < 0.999). Table 3 shows the comparison of IgG titer between the hemodialysis and control groups based on the presence of side effects (fever, headache, myalgia). The results indicate a significant relationship between IgG titer and the presence of side effects in the two groups, although no significant correlation was found based on these parameters.
| Side effect | category | IgG titer | Group | P-value | |
|---|---|---|---|---|---|
| Hemodialysis | Control | ||||
| Any type of side effect | has it | negative | 2 (11.8%) | 1 (16.7%) | 0.999 < |
| Positive | 15 (88.2%) | 5 (83.3%) | |||
| does not have | negative | 0 (0%) | 2 (7.15) | 0.519 | |
| Positive | 17 (100%) | 26 (92.9%) | |||
| Fever | has it | negative | 0 (0%) | 1 (20%) | 0.357 |
| Positive | 9 (100%) | 4 (805) | |||
| does not have | negative | 2 (8%) | 2 (6.9%) | 0.999 < | |
| Positive | 23 (92%) | 27 (93.1%) | |||
| Headache | has it | negative | 0 (0%) | 1 (33.3%) | 0.3 |
| Positive | 7 (100%) | 2 (66.7%) | |||
| does not have | negative | 2 (7.4%) | 2 (6.5%) | 0.99 < | |
| Positive | 25 (92.6%) | 29 (93.5%) | |||
| myalgia | has it | negative | 0 (0%) | 1 (50%) | 0.167 |
| Positive | 10 (100%) | 1 (50%) | |||
| does not have | negative | 2 (8.3%) | 2 (6.35) | 0.999 < | |
| Positive | 22 (91.7%) | 30 (93.8%) | |||
Table: 3 Comparison of IgG titer in two hemodialysis and control groups based on side effects.
In Table 4, the IgG titer in the hemodialysis and control groups was compared in relation to prior infection with the coronavirus and hospitalization due to COVID-19. According to the results, there was no significant correlation between IgG titer and the two investigated groups based on these parameters.
| Variable | category | IgG titer | Group | P-value | |
|---|---|---|---|---|---|
| Hemodialysis | Control | ||||
| History of covid-19 infection before the vaccine | has it | negative | 2 (10.5%) | 2 (16.7%) | 0.63 |
| Positive | 17 (89.5%) | 10 (83.3%) | |||
| does not have | negative | 0 (0%) | 1 (4.5%) | 0.999 < | |
| Positive | 15 (1005) | 21 (95.5%) | |||
| Hospitalization due to covid-19 | has it | negative | 0 (0%) | 0 (0%) | - |
| Positive | 5 (100%) | 2 (1005) | |||
| does not have | negative | 2 (14.3%) | 2 (20%) | 0.999 < | |
| Positive | 12 (85.7%) | 8 (80%) | |||
Table: 4 Comparison of IgG titer in two hemodialysis and control groups based on Covid-19 infection before vaccination and hospitalization.
The study examined the relationship between IgG titer and two factors: the time elapsed since the first hemodialysis session and the time elapsed since the last dose of injected vaccine. The results revealed no significant correlation between the elapsed time and IgG titer (P=0.699 and P=0.323, respectively), Figure 2.
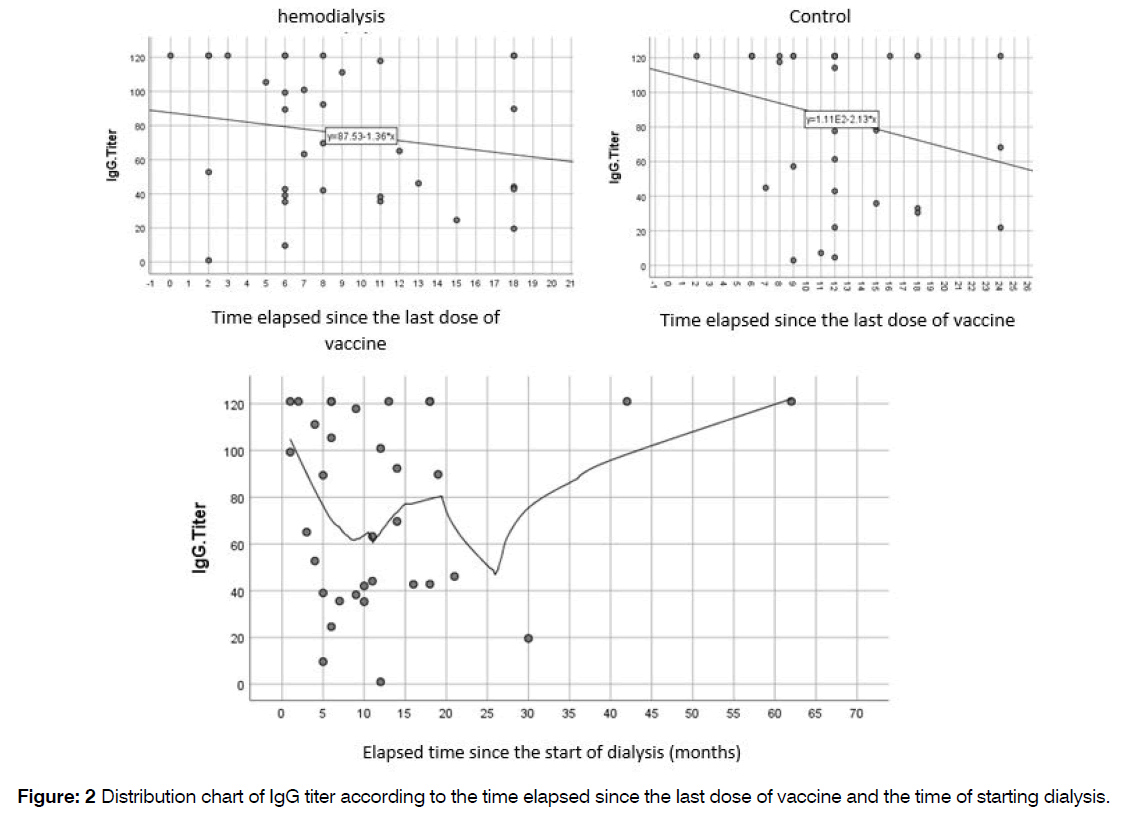
Figure 2: Distribution chart of IgG titer according to the time elapsed since the last dose of vaccine and the time of starting dialysis.
Table 5 demonstrates that there is a negative correlation coefficient between the time elapsed since the last vaccine dose and IgG titer. As the time since the last vaccine increases, the IgG titer shows a decreasing trend, with the control group exhibiting a steeper decline compared to the hemodialysis group. Similarly, the correlation coefficient for the time elapsed since the start of dialysis and IgG titer is also negative, indicating a downward trend in IgG titer over time.
| Group | The correlation coefficient | P-value |
|---|---|---|
| Hemodialysis | 0.198 - | 0.262 |
| Witness | 0.233 - | 0.184 |
Table: 5 Presents a comparison of the correlation between the times elapsed since the last vaccine dose and IgG titer.
Discussion
The mortality rate in hemodialysis patients is higher compared to the general population due to uremia and a high prevalence of co-morbidities. Moreover, this vulnerable population is particularly susceptible to infectious diseases, leading to hospitalization and mortality, primarily due to impaired innate and adaptive immunity. Given the ongoing COVID-19 pandemic and the potential for a diminished antibody response to COVID-19 vaccination in dialysis patients, our study aimed to assess the levels of IgG antibodies in these patients who received the COVID-19 vaccine and compare them with healthy individuals.
Within the hemodialysis group, 76.5% had underlying diseases, with hypertension being the most prevalent while Obstructive Airway Disease (OAD) was the least common. In contrast, among the control group, 58.8% had underlying diseases, with hypertension being the most prevalent and OAD and stroke being the least prevalent. Neither group included cases of chemotherapy, radiotherapy, or immunodeficiency. The most commonly administered vaccine in both groups for the first and second doses was Sinopharm (64.7% in the hemodialysis group and 67.6% in the control group for the first dose, and 64.7% in the hemodialysis group and 70.6% in the control group for the second dose). In terms of the third dose, 38.2% of hemodialysis patients did not receive any vaccination, while 35.3% received the Sinopharm vaccine. In the control group, 47.1% received the Sinopharm vaccine, and 35.3% did not receive any vaccination. The fourth vaccination was not administered to 88.2% of the hemodialysis group and 79.4% of the control group. Side effects were reported by 50% of hemodialysis patients, compared to only 17.6% in the control group. Regarding previous COVID-19 infections, 55.9% of the hemodialysis group and 35.3% of the control group had a history of contracting COVID-19 before receiving vaccination.
In terms of demographic parameters, no significant differences were observed between the hemodialysis and control groups based on gender (P=0.510) or age (P=0.268). A comparison of IgG titers based on gender showed that in the hemodialysis group, two women had negative titers, while 15 men and 17 women had positive titers. In the control group, two men and one woman had negative titers, while 21 men and 10 women had positive titers. However, no significant correlation was found between IgG titer levels and gender in either the hemodialysis or control groups. Notarte et al.12 reported in their study that older individuals and males exhibited a lower humoral immune response, whereas Pani et al. [13] found that women were more likely to have higher antibody titers. These findings differ from our study, potentially attributed to variations in sample size, study design, and the inclusion of meta-analysis.
Another comparison analyzed IgG titers based on age. In the age group below 40 years, one person in the hemodialysis group had a negative titer, while nine individuals had positive titers. Similarly, in the control group, no negative titers were observed, and 18 individuals in the hemodialysis group and 12 individuals in the control group exhibited positive titers in the age range of 40-65 years. Among individuals above 65 years old, 7.1% in the hemodialysis group and 16.7% in the control group had negative titers, while 92.9% and 83.3% had positive titers, respectively. No significant relationship between IgG titer levels and age was found in both the hemodialysis and control groups. Attias et al. [14] examined 378 samples and observed that individuals over 70 years old were less likely to reach seropositivity during the last follow-up. Additionally, Grupper et al. [11] identified a significant inverse correlation between age and IgG levels in both groups, with older individuals and those in the dialysis group having a higher likelihood of being in the lower quartile compared to the control group. Mavrovouniotis et al. [15] also reported a weaker immune response in association with older age. These results differ from our study and may be attributed to differences in sample size.
By the assessment of behavioral parameters, no significant difference was observed between the two groups regarding smoking and alcohol consumption. However, the hemodialysis patients exhibited a significantly lower level of physical exercise compared to the control group (P=0.029), with 91.2% of hemodialysis patients engaging in less than three days of exercise per week, whereas the percentage was 64.7% in the control group. Additionally, the amount of sleep was significantly lower in the hemodialysis group compared to the control group (P=0.03), as nearly 62% of hemodialysis patients reported sleeping less than six hours, while this percentage was 32% in the control group. Nevertheless, there was no significant correlation between IgG titer and behavioral parameters such as exercise, smoking, alcohol consumption, and sleep patterns. In a study by Toda et al. [16], the antibody titer in the dialysis patient group was significantly lower than in the control group. Within the dialysis group, age and smoking history were identified as independent factors significantly associated with antibody titer, which differs from the results of our study potentially due to the relatively low percentage of smokers in our sample (hemodialysis: 41% and control: 26%).
The presence or absence of underlying diseases among the patients was investigated, including hyperlipidemia, diabetes mellitus, hypertension, ischemic heart disease (IHD), stroke, chronic obstructive airway disease (OAD), hypothyroidism, hyperthyroidism, single kidney, serous kidney disease, liver disease, history of organ transplant, chemotherapy, radiotherapy, and immunodeficiency. No significant difference was found between the hemodialysis and control groups in terms of underlying diseases (P=0.120). However, hypertension and hypothyroidism were significantly more prevalent in the hemodialysis group compared to the control group (P=0.006 and P=0.046, respectively: 55.9% vs. 23.5% for hypertension and 6.6% vs. 2.9% for hypothyroidism). When comparing IgG titers based on the presence of underlying diseases and their types, no significant relationship was found between the presence of underlying diseases (hyperlipidemia, diabetes mellitus, and hypertension) and IgG titer. In the study by Mavrovouniotis et al.15, factors inversely correlated with immune response in dialysis patients included immunosuppressive treatment, older age, comorbidities, longer duration of hemodialysis treatment, and higher body mass index.
The study also examined the presence or absence of side effects related to the COVID-19 vaccine and their types in both the hemodialysis and control groups. A significant difference in side effects was observed between the two groups (P<0.05), with the hemodialysis group experiencing more side effects compared to the control group (50% vs. 17.6%). Notably, paresthesia, myalgia, and arthralgia were significantly more prevalent in the hemodialysis group than in the control group (P<0.05). When assessing the relationship between IgG titers and the presence of side effects and their types, no significant relationship was found based on the presence of side effects, fever, headache, and myalgia. However, in Yoshifuji et al.’s study [17], the incidence of fever and nausea after the second vaccination was significantly higher in the hemodialysis group (P=0.039 and P=0.2). Furthermore, individuals with fever in both groups exhibited significantly higher antibody titers compared to individuals without fever (P=0.0383 and P=0.0096), which contradicts our study’s findings. These discrepancies may be attributed to differences in measuring body temperature or the inclusion of other factors indicating fever in our study population.
The history of COVID-19 infection prior to vaccination and hospitalization due to COVID-19 was investigated in hemodialysis patients and controls. No significant difference was found between the two groups regarding the history of COVID-19.
Then, the association between these parameters and IgG titer was assessed, and no significant relationship was found between IgG titer and the history of COVID-19 infection prior to vaccination or hospitalization due to COVID-19 (P<0.999). In Mavrovouniotis’s study [15], previous infection with COVID-19 resulted in a more favorable immune response in both dialysis and nondialysis patients. However, this finding contradicts Notarte’s study [12], which linked sero-negativity to a lower humoral immune response. The discrepancy could be due to the extended time gap between infection and antibody titer examination in our study or potential misdiagnoses of COVID-19 in our patient cohort.
As the final parameters, the time elapsed since the last vaccine dose in both hemodialysis patients and controls, and the time elapsed since the initiation of dialysis in hemodialysis patients, were evaluated. No significant relationship was found in either case (P=0.323 and P=0.699, respectively). Furthermore, the correlation coefficient between IgG titer and the time since the last administered vaccine dose was calculated as (-0.198) in the hemodialysis group and (-0.233) in the control group, indicating an inverse correlation between these two parameters. Notably, the rate of titer reduction was higher in the control group. Likewise, the correlation coefficient between the time since the start of hemodialysis and IgG titer was also negatively calculated. In Mavrovouniotis et al.’s study [15], a negative correlation was observed between immune response and the duration of dialysis treatment, which aligns with the findings of our study. Yoshifuji et al. [17] reported that dialysis duration was a significant independent factor affecting SARS-CoV-2 IgG titers two weeks after the second vaccination in the hemodialysis group (P=0.002), with longer dialysis duration leading to higher maximal titers. However, this finding conflicts with our study. Additionally, mentioned study found significantly lower SARS-CoV-2 IgG titers in the hemodialysis group compared to the control group three weeks after the first vaccination (P<0.00001), two weeks after the second vaccination (P=0.0002), and three months after the second vaccination (P=0.045), consistent with the current study.
Conclusion
The results of this study demonstrate that IgG titers against the S1 antigen did not significantly differ in hemodialysis patients and controls who received at least two doses of the COVID-19 vaccine, indicating a favorable response in both groups. Moreover, IgG-based immunity was not associated with age, gender, underlying diseases, vaccine side effects, behavioral parameters, duration since the last vaccine dose, or duration since the initiation of dialysis.
References
- Jin H, Fang W, Zhu M, Yu Z, Fang Y, Yan H, et al. Urgent-start peritoneal dialysis and hemodialysis in ESRD patients: complications and outcomes. PLoS One. 2016;11(11):e0166181.
- Son YJ, Choi KS, Park YR, Bae JS, Lee JB. Depression, symptoms and the quality of life in patients on hemodialysis for end-stage renal disease. Am J Nephrol. 2008;29(1):36-42.
- Chauhan R, Mendonca S. Adequacy of twice weekly hemodialysis in end stage renal disease patients at a tertiary care dialysis centre. Indian J Nephrol. 2015;25(6):329.
- Ciotti M, Ciccozzi M, Terrinoni A, Jiang WC, Wang CB, Bernardini S. The COVID-19 pandemic. Crit Rev Clin Lab Sci. 2020;57(6):365-88.
- Al-Haideri M, Mohammad TA, Darvishzadehdeldari S, Karbasi Z, Alimohammadi M, Faramarzi F, et al. Immunogenicity of COVID-19 vaccines in adult patients with autoimmune inflammatory rheumatic diseases: A systematic review and meta-analysis. Int J Rheum Dis. 2023.
- Al-Haideri MT, Mannani R, Kaboli R, Gharebakhshi F, Darvishzadehdeldari S, Tahmasebi S, et al. The effects of methotrexate on the immune responses to the COVID-19 vaccines in the patients with immune-mediated inflammatory disease: A systematic review of clinical evidence. Transpl Immunol. 2023:101858.
- Tang B, Li S, Xiong Y, Tian M, Yu J, Xu L, et al. COVID-19 pneumonia in a hemodialysis patient. Kidney medicine. 2020;2(3):354-8.
- Suri RS, Antonsen JE, Banks CA, Clark DA, Davison SN, Frenette CH, et al. Management of outpatient hemodialysis during the COVID-19 pandemic: recommendations from the Canadian Society of Nephrology COVID-19 Rapid Response Team. Can j kidney health dis. 2020;7:2054358120938564.
- Yen CC, Lin SY, Chen SC, Chiu YW, Chang JM, Hwang SJ. COVID-19 vaccines in patients with maintenance hemodialysis. J Pers Med. 2021;11(8):789.
- Dekervel M, Henry N, Torreggiani M, Pouteau LM, Imiela JP, Mellaza C, et al. Humoral response to a third injection of BNT162b2 vaccine in patients on maintenance haemodialysis. Clin Kidney J. 2021;14(11):2349-55.
- Grupper A, Sharon N, Finn T, Cohen R, Israel M, Agbaria A, et al. Humoral response to the Pfizer BNT162b2 vaccine in patients undergoing maintenance hemodialysis. Clin J Am Soc Nephrol. 2021;16(7):1037.
- Notarte KI, Ver AT, Velasco JV, Pastrana A, Catahay JA, Salvagno GL, et al. Effects of age, sex, serostatus, and underlying comorbidities on humoral response post-SARS-CoV-2 Pfizer-BioNTech mRNA vaccination: a systematic review. Crit Rev Clin Lab Sci. 2022;59(6):373-90.
- Pani A, Cento V, Vismara C, Campisi D, Di Ruscio F, Romandini A, et al. Results of the RENAISSANCE Study: REsponse to BNT162b2 COVID-19 vacciNe—short-And long-term Immune reSponSe evAluatioN in health Care workErs. Mayo Clin Proc. Elsevier. 2021
- Attias P, Sakhi H, Rieu P, Soorkia A, Assayag D, Bouhroum S, et al. Antibody response to the BNT162b2 vaccine in maintenance hemodialysis patients. Kidney Int. 2021;99(6):1490-2.
- Mavrovouniotis I, Fylaktou A, Stagou M, Ouranos K, Lioulios G, Evgenikaki E, et al. Cellular and Humoral Responses in Dialysis Patients after Vaccination with the BNT162b2 or mRNA-1273 Vaccines. Life. 2023;13(2):474.
- Toda M, Yoshifuji A, Kikuchi K, Koinuma M, Komatsu M, Fujii K, et al. Factors associated with SARS-CoV-2 antibody titers and prognosis of breakthrough infection in hemodialysis patients. Clin Exp Nephrol. 2022;26(6):571-80.
- Yoshifuji A, Toda M, Ryuzaki M, Kikuchi K, Kawai T, Sakai K, et al. Investigation for the efficacy of COVID-19 vaccine in Japanese CKD patients treated with hemodialysis. Ren Replace Ther. 2022;8(1):39.
1Department of Infectious Diseases, Zanjan University of Medical Sciences, Zanjan, Iran
2Department of Internal Medicine, Zanjan University of Medical Sciences, Zanjan, Iran
3MD, Zanjan University of Medical Sciences, Zanjan, Iran
4Department of Genetics and Molecular Medicine, School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran
Send correspondence to:
Hosein Dinmohammadi
Department of Genetics and Molecular Medicine, School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran, Email: h.dinmohammadi.700@gmail.com
Paper submitted on October 27, 2023; and Accepted on November 17, 2023
Citation: Dinmohammadi H. Investigation of IgG Titers in Hemodialysis Patients and Controls Following Administration of the COVID-19 Vaccine. Int Tinnitus J. 2023;27(2):183-190.


