The International Tinnitus Journal
Official Journal of the Neurootological and Equilibriometric Society
Official Journal of the Brazil Federal District Otorhinolaryngologist Society
ISSN: 0946-5448

Google scholar citation report
Citations : 12717
The International Tinnitus Journal received 12717 citations as per google scholar report
The International Tinnitus Journal peer review process verified at publons
Indexed In
- Excerpta Medica
- Scimago
- SCOPUS
- Publons
- EMBASE
- Google Scholar
- Euro Pub
- CAS Source Index (CASSI)
- Index Medicus
- Medline
- PubMed
- UGC
- EBSCO
Volume 27, Issue 2 / December 2023
Research Article Pages:211-216
10.5935/0946-5448.20230032
Prevalence and antimicrobial resistance of bacterial agents isolated from the cases of dental caries
Authors: Siavash Asadi Paein Lamooki, Faezeh Sadeghi Heris, Amirhossein Fathi, Negin Aminianpour, Zahra Jandaghian, Maryam Alipanahi Ramandi*
PDF
Abstract
Dental caries are mainly occur owing to the presence and activity of bacterial agents. The present study was done to assess the prevalence and antibiotic resistance of bacterial strains isolated from the cases of dental caries. Fifty patients with approved dental carries were included in the study. Sampling from the site of dental caries was done using the sterile swab. Swabs were transferred to laboratory and subjected to microbial culture. Species identification of bacteria was done using biochemical test. Bacterial isolates were subjected to disk diffusion to assess their antimicrobial resistance. S. aureus (40%) harboured the highest rate of contamination, while S. oralis (16%) and E. aerogenes (10%) harbored the lowest. S. aureus and S. mutans (6%) harbored the highest distribution amongst the cases of mix infections, while S. aureus and S. oralis (2%) harbnored the lowest. S. aureus strains harbored the highest rate of resistance toward tetracycline (90%), penicillin (75%), ampicillin (75%), amoxicillin (60%), and erythromycin (60%). E. coli strains harbored the highest rate of resistance toward tetracycline (90%), gentamicin (80%), ampicillin (70%), and erythromycin (70%). S. mutans strains harbored the highest rate of resistance toward tetracycline (93.33%), ampicillin (86.66%), penicillin (80%), amoxicillin (80%), and erythromycin (80%). S. oralis strains harbored the highest rate of resistance toward tetracycline (100%), ampicillin (75%), penicillin (62.50%), and amoxicillin (62.50%). E. aerogenes strains harbored the highest rate of resistance toward tetracycline (80%), gentamicin (80%), and ampicillin (80%). S. aureus bacteria isolated from dental caries harbored the highest rate of MDR. Distribution of resistance against more than 3 antimicrobial agents amongst the S. aureus, E. coli, S. mutans, S. oralis, and E. aerogenes bacteria isolated from the cases of dental caries was 90%, 60%, 80%, 62.50%, and 80%, respectively. Application of disk diffuin can help practitioners to reduce the rate of resistance in bacteria responsible for dental caries.
Keywords: Dental caries, Bacteria, Antibiotic resistance, Prevalence.
Introduction
Dental caries (also recognized as dental cavities or tooth decay) is the most mutual non-communicable disease of the oral cavity globally. Severe dental caries affects human health and frequently causes pain and infection, which may bring about tooth extraction1. It is an expensive disease to treat, overriding 5–10% of healthcare budgets in developed countries, and is among the chief reasons for children hospitalization in some high-income countries2. As a result, it is essential to determine all epidemiological aspects, etiological agents, and routine ways to treat subsequent infections.
High amounts of nutritional materials, epithelial debris, and secretions caused the mouth to be a favorable environment for growth and proliferation of bacteria3. In this regard, Staphylococcus, Streptococcus, Escherichia, and Enterobacter species are the most significant and frequent species isolated from the cases of dental caries4,5.
Stereptococcus mutans (S. mutans) and S. oralis, Staphylococcus aureus (S. aureus), Enterobacter aerogenes, and Escherichia coli (E. coli) are considered to be the most common causes of bacterial infections in the oral cavity6-10. These bacteria mainly harbored high rate of resistance against commonly-used antimicrobials, especially penicillin, aminoglycosides, macrolides, cephalosporin, quinolones, and tetracyclines11-16. This issue increased the importance of oral infections caused by these bacteria, prolongs the hospitalized persons, and increased the costs of treatment17.
Due to the lack of epidemiological, dental and microbiological studies in this field, the present study was conducted to evaluate the frequency of bacterial agents effective in causing dental caries and assess their antimicrobial resistance pattern.
Materials and Methods
Media and chemical reagents
All culture medica and chemical reagents were purchased from Merck Company (Merck, Germany). Antimicrobial disks were purchased from the Oxoid company (Oxoid, UK).
Samples
The current cross sectional and descriptive survey was done on summer of 2023. A total of 50 patients who were referred to private dentistry clinics owing to the dental caries were included in the research. Disposable cotton swabs with standard protocol were applied for sampling from the site of dental caries. No cross contamination was occurred during the sampling. All samples were transferred to laboratory within 2 hr after collection using sterile refrigerator (4 ± 1 °C)18,19.
Bacterial isolation and identification
The dental caries samples which were taken using sterile cotton swab was cultured into different tubes containing chocolate agar, 5% sheep blood agar, and a selective medium. All media were then transferred to the private microbiology laboratory. All media were incubated at 37°C and 42 °C for 24 to 48 h. After Gram staining and microscopy, different biochemical tests were performed to identify bacterial strains. Different biochemical examinations, including oxidase, catalase, urease, indole, Methyl Red, Voges Proskauer, Simon Citrate, Coagulase, and starch tests were applied. Analytical Profile Index (API 20E) (BioMeriouxVitek, Inc., MO, USA) system was used to identify bacteria20.
Antimicrobial resistance
CLSI procedures were applied to assess the antimicrobial resistance of isolates. Mueller–Hinton agar (Merck, Germany) was applied. Diverse antimicrobial disks, including penicillin (10 μg/disk), ampicillin (10 μg/ disk), amoxicillin (25 μg/disk), ceftriaxone (30 μg/ disk), vancomycin (30 μg/disk), azithromycin (15 μg/ disk), erythromycin (15 μg/disk), metronidazole (5 μg/ disk), gentamicin (10 μg/disk), rifampin (30 µg/disk), and tetracycline (30 μg/disk), were placed on media. Microbial media with placed disks were incubated (35°C for 24 h). Aerobic and anaerobic conditions were applied according to the targeted bacteria. Guidelines of the CLSI were applied for susceptibility analysis21,22.
Data analysis
Data were subjected to Microsoft Office Excel (version 15; Microsoft Corp., Redmond, WA, USA)23. The statistical analysis was performed employing the SPSS 21.0 software (SPSS Inc., Chicago, IL, USA)24. Chi-square test and Fisher’s exact two-tailed test were applied to measure any significant relationship. P-value <0.05 was considered as a significant numerical level25,26.
Results
Distribution of bacterial strains
Table 1 shows the bacterial agents isolated from the total of 50 cases of dental caries. S. aureus (40%) harbored the highest rate of contamination, while S. oralis (16%) and E. aerogenes (10%) harbored the lowest (P <0.05).
| Bacterial distribution (%) | ||||||
|---|---|---|---|---|---|---|
| Samples | N collected | Staphylococcus aureus | Escherichia coli | Streptococcus mutans | Stereptococcus oralis | Enterobacter aerogenes |
| Dental caries |
50 | 20 (40) | 10 (20) | 15 (30) | 8 (16) | 5 (10) |
Table 1: Distribution of the bacterial agents isolated from the total of 50 cases of dental caries.
Distribution of mix infections
Figure 1 shows the mix infections distribution amongst the examined samples. Rendering obtained findings, S. aureus and S. mutans (6%) harbored the highest distribution amongst the cases of mix infections, while S. aureus and S. oralis (2%) harbored the lowest (P <0.05).
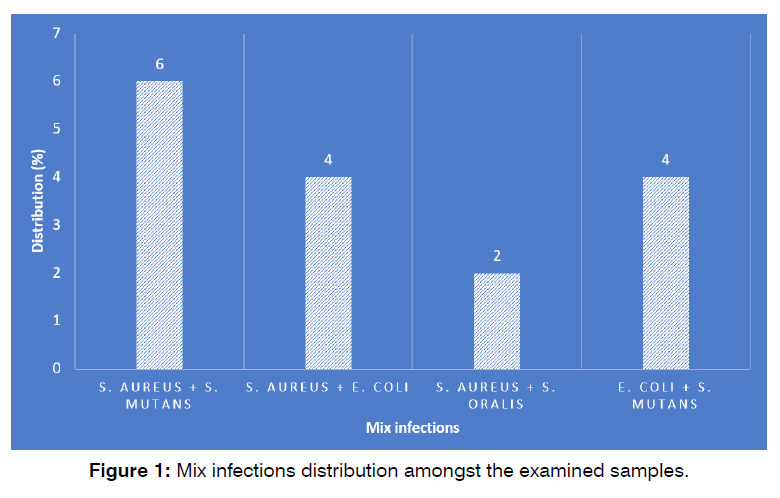
Figure 1: Number of cases succeeded and failed in the study and control groups.
Antimicrobial resistance
figure 2 shows the antimicrobial resistance of examined bacterial agents against commonly-used antimicrobial agents in dentistry. S. aureus strains harbored the highest rate of resistance toward tetracycline (90%), penicillin (75%), ampicillin (75%), amoxicillin (60%), and erythromycin (60%). E. coli strains harbored the highest rate of resistance toward tetracycline (90%), gentamicin (80%), ampicillin (70%), and erythromycin (70%). S. mutans strains harbored the highest rate of resistance toward tetracycline (93.33%), ampicillin (86.66%), penicillin (80%), amoxicillin (80%), and erythromycin (80%). S. oralis strains harbored the highest rate of resistance toward tetracycline (100%), ampicillin (75%), penicillin (62.50%), and amoxicillin (62.50%). E. aerogenes strains harbored the highest rate of resistance toward tetracycline (80%), gentamicin (80%), and ampicillin (80%). The lowest rate of resistance in S. aureus, E. coli, S. mutans, S. oralis, and E. aerogenes strains were obtained against azithromycin (20%), azithromycin and vancomycin (20% each), azithromycin and vancomycin (26.66% each), azithromycin and vancomycin (12.50% each), and finally ceftriaxone, vancomycin, and rifampin (20% each). Statistically significant difference was obtained between type of bacteria and prevalence of antibiotic resistance (P <0.05).
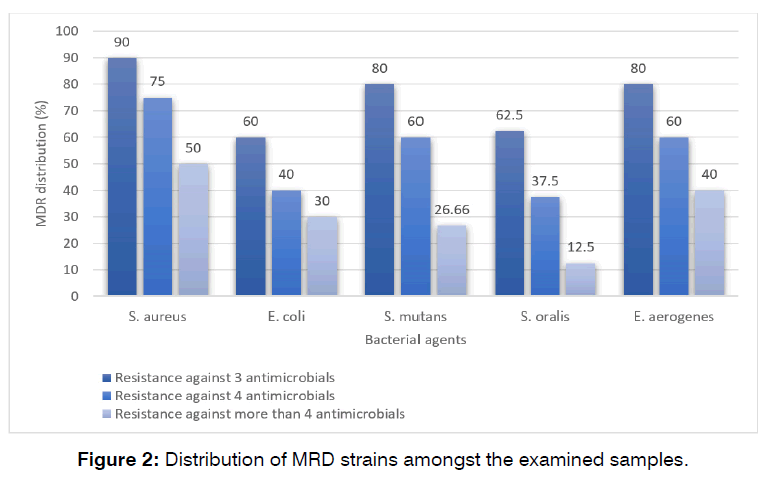
Figure 2: Number of cases succeeded and failed in the study and control groups.
*P10: penicillin (10 μg/disk), A10: ampicillin (10 μg/disk), Ax25: amoxicillin (25 μg/disk), Cft: ceftriaxone (30 μg/ disk), V30: vancomycin (30 μg/disk), Az: azithromycin (15 μg/disk), E15: erythromycin (15 μg/disk), Met: metronidazole (5 μg/disk), G10: gentamicin (10 μg/disk), Rif: rifampin (30 µg/disk), T30: tetracycline (30 μg/disk) Table 2.
| Bacteria (N. isolated) | Antimicrobial resistance (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P10 | A10 | Ax25 | Cft | V30 | Az | E15 | Met | G10 | Rif | T30 | |
| Staphylococcus aureus (20) | 15 (75) | 15 (75) | 12 (60) | 8 (40) | 5 (25) | 4 (20) | 12 (60) | 7 (35) | 10 (50) | 10 (50) | 18 (90) |
| Escherichia coli (10) | 5 (50) | 7 (70) | 6 (60) | 4 (40) | 2 (20) | 2 (20) | 7 (70) | 3 (30) | 8 (80) | 4 (40) | 9 (90) |
| Streptococcus mutans (15) | 12 (80) | 13 (86.66) | 12 (80) | 10 (66.66) | 4 (26.66) | 4 (26.66) | 12 (80) | 10 (66.66) | 7 (46.66) | 8 (53.33) | 14 (93.33) |
| Stereptococcus oralis (8) | 5 (62.50) | 6 (75) | 5 (62.50) | 2 (25) | 1 (12.50) | 1 (12.50) | 3 (37.50) | 2 (25) | 3 (37.50) | 3 (37.50) | 8 (100) |
| Enterobacter aerogenes (5) | 2 (40) | 4 (80) | 3 (60) | 1 (20) | 1 (20) | - | 3 (60) | 2 (40) | 4 (80) | 1 (20) | 4 (80) |
Table 2: Antimicrobial resistance of examined bacterial agents against commonly-used antimicrobial agents in dentistry.
Multi-drug resistance distribution
Figure 2 shows the distribution of MRD strains amongst the examined samples. MDR strains were determined as those which had simultaneous resistance to at least 3 antimicrobial agents. S. aureus bacteria isolated from dental caries harbored the highest rate of MDR. Distribution of resistance against more than 3 antimicrobial agents amongst the S. aureus, E. coli, S. mutans, S. oralis, and E. aerogenes bacteria isolated from the cases of dental caries was 90%, 60%, 80%, 62.50%, and 80%, respectively.
Discussion
In despite of all advances occurred in dentistry27-30, dental caries remain an important destroying issue of teeth and is a major problem for all people, especially in old age.
The present study showed that S. aureus, S. mutans, S. oralis, E. aerogenes, and E. coli were isolated from the cases of dental caries, with the highest distribution of S. aureus and S. mutans. Similar to tis research, S. aureus and S. mutans were also predominant bacterial agents responsible for dental caries, dental plaques, and other related infections of the oral cavity in diverse researches31-38. However, some surveys highlighted the role of other streptococcal species and E. coli, and E. aerogenes in the occurrence of dental caries, dental plaques, and other related infections of the oral cavity39-41.
Daniyan and Abalaka (2011)42 stated that the distribution of S. aureus and S. mutans amongst the dental caries samples were 53.40% and 39.70%, respectively. In dental plaques, total distribution of S. aureus, S. mutans, and E. coli was 15%, 19%, and 10%, respectively43. Similar to this, their high distribution in the cases of maxillofacial surgery were also reported44.
Isolates harbored high resistance toward tetracycline, penicillin, ampicillin, amoxicillin, and erythromycin. Gramnegative isolates also harbored the high rate of resistance toward gentamicin. High antimicrobial resistance of Gram-negative and Gram-positive bacteria isolated from the dental caries against tetracycline, penicillin, ampicillin, amoxicillin, gentamicin, and erythromycin was also reported by 45-48.
Jassam et al. (2022)49 described that S. mutans isolates were resistant to penicillin (82.2%) and highly sensitive to amoxicillin (86.6%) and ciprofloxacin (71.1%). They showed that S. oralis strains were resistant to imperium (100%), and highly sensitive to gentamycin, ciprofloxacin, cefotaxime, and amoxicillin (100%). S. epidermidis was highly resistant to tetracycline at a rate of (88.40%) and highly sensitive to amoxicillin at the same rate (88.40% each). E. coli was highly sensitive to gentamycin, imperium, amoxicillin and vancomycin (91.40%). Yadav et al. (2015)49 stated that the prevalence of resistance of S. mutans bacteria isolated from dental plaque samples against ampicillin, ceftriaxone, ciprofloxacin, cotrimoxazole, erythromycin, gentamicin, tetracycline, penicillin, and impanel was 26.92% 41.56%, 22.30%, 20% ,0%, 0%, 60.76%, 60.15, and 0%, respectively. Resistance rate of S. aureus against the above-mentioned agents was 61.70%, 2021%, 21.24%, 50%, 46.80%, 58.51%, 86.17%, 91.48% and 0%, respectively, which was similar to our findings.
Totally, this survey is one of the first reports of identification of antimicrobial resistance of pathogenic bacteria responsible for dental caries. Findings are limited to the low number of isolated bacteria, lack of demographical characters of the studied population and also absence of the determination of the history of gastrointestinal disorders among patients.
Conclusion
Totally, S. aureus, S. mutans, S. oralis, E. coli, and E. aerogenes were isolated from the cases of dental caries. Among them, S. aureus and S. mutans had the highest distribution. Most of bacterial isolates were resistant against tetracycline, ampicillin, amoxicillin, and gentamicin, erythromycin, and penicillin antimicrobials. This matter may show the low efficacy of these antimicrobial agents for treatment and control of infections after dental caries. Application of disk diffuin can reduce the risk of the occurrence of antibiotic resistance amongst the dental bacteria. Supplementary researches can help to learn more about the role of dental caries bacteria and their antibiotic resistance in the oral cavity.
References
- Pitts NB, Zero DT, Marsh PD, Ekstrand K, Weintraub JA, Ramos-Gomez F, et al. Dental caries. Nat Rev Dis Primers. 2017;3(1):1-6.
- Kidd EA, Fejerskov O. Essentials of dental caries. Oxford University Press. 2016.
- Hasslöf P, Stecksén-Blicks C. Probiotic bacteria and dental caries. Monogr Oral Sci. 2020;28:99-107.
- Karpinski TM, Szkaradkiewicz AK. Microbiology of dental caries. J Biol Earth Sci. 2013;3(1):M21-4.
- Yang Z, Cai T, Li Y, Jiang D, Luo J, Zhou Z. Oral microbial communities in 5-year-old children with versus without dental caries. BMC Oral Health. 2023;23(1):1-6.
- OmerOglou E, Karaca B, Kibar H, Haliscelik O, Kiran F. The role of microbiota-derived postbiotic mediators on biofilm formation and quorum sensing-mediated virulence of Streptococcus mutans: A perspective on preventing dental caries. Microb Pathog. 2022;164:105390.
- Ahmed NA, Tariq P, Naim A. Viridans group streptococci and dental caries: An overview. Int J Biol Biotech. 2023;20(1):3-16.
- Asghar F, Bano A, Waheed F, Anjum AA, Ejaz H, Javed N. Association of exogenous factors with molecular epidemiology of Staphylococcus aureus in human oral cavity. Saudi J Biol Sci. 2023;30(4):103613.
- Alghamdi S. Isolation and identification of the oral bacteria and their characterization for bacteriocin production in the oral cavity. Saudi J Biol Sci. 2022;29(1):318-23.
- Abd Ali MA, Shareef AA. Antibacterial Activity of Silver Nanoparticles Derived from Extracellular Extract of Enterococcus aerogenes Against Dental Disease Bacteria Isolated. Regen Eng Transl Med. 2023:1-0.
- Saliminasab M, Jabbari H, Farahmand H, Asadi M, Soleimani M, Fathi A. Study of antibacterial performance of synthesized silver nanoparticles on Streptococcus mutans bacteria. J Nanomed Res. 2022;7(4):391-6.
- Mortezagholi B, Movahed E, Fathi A, Soleimani M, Forutan Mirhosseini A, Zeini N, et al. Plant-mediated synthesis of silver-doped zinc oxide nanoparticles and evaluation of their antimicrobial activity against bacteria cause tooth decay. Microsc Res Tech. 2022;85(11):3553-64.
- Khamisi N, Fathi A, Yari A. Antimicrobial resistance of Staphylococcus aureus isolated from dental plaques. Acad J Health Med Nurs. 2022;37(1):136-40.
- Fathi A, Salehi A. Antimicrobial resistance properties of Helicobacter pylori strains isolated from dental plaque and saliva samples. Acad J Health Med Nurs. 2022;37(1):29-33.
- Mirzaei K, Fathi A, Asadinejad SM, Moghadam NC. Study the antimicrobial effects of Zataria multiflora-based mouthwash on the microbial community of dental plaques isolated from children: A candidate of novel plant-based mouthwash. Acad J Health Med Nurs. 2022;37(3):58-63.
- Fathi A, Mostajeran E. Distribution and antimicrobial resistance of bacterial pathogens isolated from dental plaques.
- Safarpoor Dehkordi F, Gandomi H, Basti AA, Misaghi A, Rahimi E. Phenotypic and genotypic characterization of antibiotic resistance of methicillin-resistant Staphylococcus aureus isolated from hospital food. Antimicrob Resist Infect Control. 2017;6(1):1-1.
- Khaji L, Shahreza MH. SCCmec types in methicillin-resistant Staphylococcus aureus strains of various types of milk. Electronic J Biol. 2016;13(1).
- Shahreza MH, Rahimi E, Momtaz H. Antibiotic resistance pattern of Shiga-toxigenic Escherichia coli isolated from ready-to-eat food stuffs. Biosc Biotech Res Comm. 2017;10(2):155-9.
- Shayegani ME, Maupin PS, McGLYNN DM. Evaluation of the API 20E system for identification of nonfermentative Gram-negative bacteria. J Clin Microbiol. 1978;7(6):539-45.
- Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing; twenty-second informational supplement. M100-S21. Wayne Pa: CLSI; 2012.
- Mashak Z, Jafariaskari S, Alavi I, Sakhaei Shahreza M, Safarpoor Dehkordi F. Phenotypic and genotypic assessment of antibiotic resistance and genotyping of vacA, cagA, iceA, oipA, cagE, and babA2 alleles of Helicobacter pylori bacteria isolated from raw meat. Infect Drug Resist. 2020:257-72.
- Ranjbar R, Shahreza MH, Rahimi E, Jonaidi-Jafari N. Methicillin-resistant Staphylococcus aureus isolates from Iranian restaurant food samples: Panton-Valentine Leukocidin, SCCmec phenotypes and antimicrobial resistance. Trop J Pharm Res. 2017;16(8):1939-49.
- Ranjbar R, Shahreza MH. Prevalence, antibiotic-resistance properties and enterotoxin gene profile of Bacillus cereus strains isolated from milk-based baby foods. Trop J Pharm Res. 2017;16(8):1931-7.
- Shahreza MH, Rahimi E, Momtaz H. Shiga-toxigenic Escherichia coli in ready-to-eat food staffs: Prevalence and distribution of putative virulence factors. Microbiol Res. 2017;8(2):7244.
- Ranjbar R, Safarpoor Dehkordi F, Sakhaei Shahreza MH, Rahimi E. Prevalence, identification of virulence factors, O-serogroups and antibiotic resistance properties of Shiga-toxin producing Escherichia coli strains isolated from raw milk and traditional dairy products. Antimicrob Resist Infect Control. 2018;7(1):1-1.
- Hashemi S, Tabatabaei S, Baghaei K, Fathi A, Atash R. Long-Term Clinical Outcomes of Single Crowns or Short Fixed Partial Dentures Supported by Short (= 6 mm) Dental Implants: A Systematic Review. Eur J Dent. 2023.
- Fathpour K, Astaraki E, Zandian A, Fathi A, Mirmohammadi H. Shear bond strength of composite resins to lithium disilicate ceramics using universal bonding and different methods of surface preparation. J Dent Res. 2023;20(1):82.
- Fathi A, Barjoee SS. Glass-Ceramic and Zirconia-Based Dental Restorations; A Narrative Review on Introduction and Applications. J Coast Life Med. 2023;11:1603-9.
- Fathi A, Rismanchian M, Yazdekhasti A, Salamati M. Accuracy of tooth-implant impressions: Comparison of five different techniques. Clin Exp Dent Res. 2023.
- Javed S, Zakirulla M, Baig RU, Asif SM, Meer AB. Development of artificial neural network model for prediction of post-streptococcus mutans in dental caries. Comput Methods Programs Biomed Update. 2020;186:105198.
- Alejandra BM, Daniel OM. Virulence factors of Streptococcus mutans related to dental caries. Staphylococcus Streptococcus. 2020;11:9.
- Nomura R, Matayoshi S, Otsugu M, Kitamura T, Teramoto N, Nakano K. Contribution of severe dental caries induced by Streptococcus mutans to the pathogenicity of infective endocarditis. Infect Immun. 2020;88(7):10-128.
- Sounah SA, Madfa AA. Correlation between dental caries experience and the level of Streptococcus mutans and lactobacilli in saliva and carious teeth in a Yemeni adult population. BMC Res Notes. 2020;13(1):1-6.
- Al-Akwa AA, Zabara AQ, Al-Shamahy HA, Al-Labani MA, Al-Ghaffari KM, Al-Mortada AM, et al. Prevalence of Staphylococcus aureus in dental infections and the occurrence of MRSA in isolates. Univers J Pharm Res. 2020;5(2):23-7.
- Nasr-Eldin MA, El-Dougdoug NK, Elazab YH, Esmael A. Isolation and Characterization of Two Virulent Phages to Combat Staphylococcus aureus and Enterococcus faecalis causing Dental Caries. J Pure Appl Microbiol. 2021;15:320-34.
- Donkor ES, Kotey FC. Methicillin-resistant Staphylococcus aureus in the oral cavity: implications for antibiotic prophylaxis and surveillance. Infect Dis Ther. 2020;13:1178633720976581.
- Abd Ali MA, Shareef AA. Green Synthesis of Silver Nanoparticles by Enterobacter Aerogenes Bacteria in Combination with Antibiotics Against Multidrug Resistance Streptococcus Mitis Isolated from Oral Cavity of Some Dental Caries Patients in Misan City. Ann Romanian Soc Cell Biol. 2021:13768-89.
- Martins NB, Ferreira LA, Santos AL, de Souza RR, Oliveira WJ, de Almeida Moreira T, et al. Dermatopathy caused by enterobacter aerogenes and Pseudomonas aeruginosa in Boa constrictor amarali. Acta Scientiae Veterinariae. 2017;45:1-4.
- Mahamoud A, Chevalier J, Davin-Regli A, Barbe J. Quinoline derivatives as promising inhibitors of antibiotic efflux pump in multidrug resistant Enterobacter aerogenes isolates. Curr Drug Targets. 2006;7(7):843-7.
- Daniyan SY, Abalaka ME. Prevalence and susceptibility pattern of bacterial isolates of dental caries in a secondary health care institution, Nigeria.
- Mirzaei K, Fathi A, Asadinejad SM, Moghadam NC. Study the antimicrobial effects of Zataria multiflora-based mouthwash on the microbial community of dental plaques isolated from children: A candidate of novel plant-based mouthwash. Acad J Health Med Nurs. 2022;37(3):58-63.
- Moghadam NC, Fathi A, Salehi A, Rad MB. Distribution of bacteria isolated from the cases of maxillofacial surgery. Med balear. 2022;37(4):21-5.
- Meinen A, Reuss A, Willrich N, Feig M, Noll I, Eckmanns T, et al. Antimicrobial resistance and the spectrum of pathogens in dental and oral-maxillofacial infections in hospitals and dental practices in Germany. Front Microbiol. 2021;12:676108.
- Li X, Wang Y, Jiang X, Zeng Y, Zhao X, Washio J, et al. Investigation of drug resistance of caries-related streptococci to antimicrobial peptide GH12. Front Cell Infect Microbiol. 2022:1332.
- Rezazadeh F, Azad A, Khorami A, Modaresi F, Rezaie Z. Evaluation of Antibiotic Resistance Pattern in Dental Bacteremia Detected by Multiplex PCR Technique. Biomed Res Int. 2020.
- Kouidhi B, Zmantar T, Mahdouani K, Hentati H, Bakhrouf A. Antibiotic resistance and adhesion properties of oral Enterococci associated to dental caries. BMC Microbiol. 2011;11(1):1-7.
- Jassam RA, Abed AS, Abood ES. Antimicrobial Susceptibility Pattern of Some Pathogenic Bacteria Isolated from Dental Caries. Egypt J Chem. 2022;65(4):701-14.
- Yadav K, Prakash S, Yadav NP, Sah RS. Multi-drug resistance of bacterial isolates among dental caries patients.
1Postgraduate Student, Department of Prosthodontics, School of Dentistry, Tehran University of Medical Sciences, Tehran, Iran.
2Postgraduate Student, Department of Periodontics, School of Dentistry, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
3Dental Prosthodontics Department, Dental Materials Research Center, Isfahan University of Medical Sciences, Isfahan, Iran.
Send correspondence to:
Dr. Maryam Alipanahi Ramandi
Department of Prosthodontics, School of Dentistry, Tehran University of Medical Sciences, Karegar St, Tehran, Iran. E-mail: marym.alipanahi74@gmail.com
Paper submitted on November 07, 2023; and Accepted on November 29, 2023
Citation: Ramandi AM. Prevalence and antimicrobial resistance of bacterial agents isolated from the cases of dental caries. Int Tinnitus J. 2023;27(2):211-216.


