The International Tinnitus Journal
Official Journal of the Neurootological and Equilibriometric Society
Official Journal of the Brazil Federal District Otorhinolaryngologist Society
ISSN: 0946-5448

Google scholar citation report
Citations : 12717
The International Tinnitus Journal received 12717 citations as per google scholar report
The International Tinnitus Journal peer review process verified at publons
Indexed In
- Excerpta Medica
- Scimago
- SCOPUS
- Publons
- EMBASE
- Google Scholar
- Euro Pub
- CAS Source Index (CASSI)
- Index Medicus
- Medline
- PubMed
- UGC
- EBSCO
Volume 27, Issue 1 / June 2023
Research Article Pages:16-26
10.5935/0946-5448.20230004
Prevalence of Hearing Loss among Very Low Birth Weight (VLBW) Babies in Two Tertiary Hospitals in Malaysia
Authors: Asma Abdullah, Rebecca Wilfred, Asfa Najmi Mohamad Yusof, Wan Fazlina Wan Hashim
PDF
Abstract
Objective: This study aimed to evaluate hearing loss among very low birth weight babies in two hospitals in Malaysia.
Material and Methods: A total of 380 babies from Hospital Canselor Tuanku Muhriz (HCTM), Kuala Lumpur and Sarawak General Hospital (SGH) were recruited in this retrospective study. All babies with birthweight less than 1500grams nursed in the Neonatal Intensive Care Unit (NICU) between January 2014 till December 2019 was included in the study. Data was analysed on demography, interval taken for hearing intervention and defaulter rate. The data of patient parameters between both hospitals were analysed and association between various factors were evaluated.
Results: A total 187 Very Low Birth Weight (VLBW) Kuala Lumpur babies and 193 VLBW Sarawak babies met the inclusion and exclusion criteria, among which 10.1% and 10.9% had SNHL in Kuala Lumpur and Sarawak respectively. CHL was reported among 8.6% Kuala Lumpur and 14% of Sarawak babies. When studied on the different types and degrees of hearing loss, 2.6% of Kuala Lumpur babies born less than 28 Weeks Gestation Age (WGA) had moderate SNHL and 2.0% of Sarawak babies had profound SNHL. In this study only gestational age (week) (p=0.003) and dysmorphism (p<0.001) were statistically significant to be associated with hearing loss.
Conclusion: The prevalence of hearing loss among VLBW babies in Kuala Lumpur was 20.3% and 24.8% in Sarawak. Gestational age (p=0.044) and presence of dysmorphism (p<0.001) were found to have statistically significant association with prevalence of hearing loss. The defaulter rate at Kuala Lumpur was 52.6% and 42.3% in Sarawak.
Keywords: Tinnitus, Hearing loss, Low birth weight.
Introduction
Prevalence of hearing loss among full term newborn is 1 to 3 in 1000 live births, however it is much higher in preterm babies, 2 to 4 in 100 live births [1-3]. Hearing loss has been a major issue faced by very low birth weight babies, as two thirds of these babies are born premature. It is unclear whether having a younger gestational age or lower birth weight are the implicating right factor, or presence of other attributes render these group of babies to be more vulnerable to hearing deficit4. The Joint Committee on Infant Hearing (JCIH) have constructed a set of risk factors for infants at risk of hearing loss and it is found that between 10 to 30 % of children will have one of these factors at birth, that includes VLBW [4]. Disabling hearing loss therefore refers to hearing loss greater than 30 decibels (dB) in the better hearing ear. Failure to diagnose this condition will place the innocent baby at a disadvantage academically and socially in the future as a result of consequences of a hearing loss gone undiagnosed. During recent years, the mortality rate of high-risk infants nursed in the Neonatal Intensive Care Unit (NICU) has gradually decreased with advances in medicine. These groups of infants that survive neonatal complications inadvertently become more prone to developmental abnormalities, including peripheral and/or central hearing impairment. In this study we identify the prevalence of hearing loss in VLBW infants, its co-existing risk factor profiles, the mode of intervention among and the duration taken to intervene.
Methodology
This was a retrospective comparative study conducted from January 2014 to December 2019 at two tertiary care hospitals in territory of Kuala Lumpur and the state of Sarawak, Malaysia respectively. This study received approval from the University Research Ethic Committee (UKM JEP-2020-557. We studied data of all babies nursed in the Neonatal Intensive Care Unit (NICU) weighing less than 1500 grams. A total of 187 babies from Hospital Canselor Tuanku Muhriz (HCTM) Kuala Lumpur (KL) and 193 babies from Sarawak General Hospital (SGH) were included in this study Figure 1. Exclusion criteria included the incomplete data in the patients’ record. The risk factors of hearing loss were recorded. The summarized methodology is represented in Figure 2.
Figure 1: Data was analyzed by using Statistical Package.
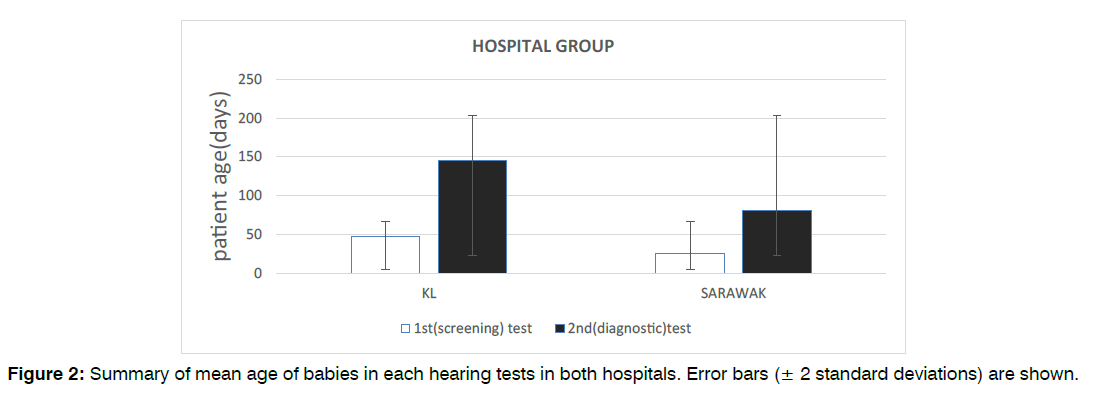
Figure 2: Summary of mean age of babies in each hearing tests in both hospitals. Error bars (± 2 standard deviations) are shown.
Those babies underwent first stage of hearing screening via portable automated Otoacoustic Emissions (OAE). Irrespective of the OAE result, all the babies underwent diagnostic Auditory Brainstem Response (ABR). This was performed in Audiology clinic in both centers. The babies that missed appointment for the ABR were given a new date. Latencies of ABR waves I, III and V were measured. Based on the results, babies were diagnosed with hearing deficit were classified as mild (21–40 dB), moderate (41– 70 dB), severe (71–90 dB) and profound (>90 dB). Data was analyzed by using Statistical Package for the Social Sciences version 28 (IBM SPSS Statistics 28) and the Microsoft Excel 2011 version 16.48.
Statistical Analysis: The Statistical Package for the Social Sciences version 28 (IBM SPSS Statistics 28) and the Microsoft Excel 2011 version 16.48 for the Macintosh platform were utilized for statistical analysis.
The baseline demographic data were expressed as mean and standard deviation for continuous data or frequency and percentages for categorical data.
The comparisons of various patient parameters between both hospitals were analyzed with Mann-Whitney U test for numerical data or Wilcoxon Ranke Sum Test for ordinal data. A value of p<0.05 was considered significant.
Chi-squared test was used for univariate analysis to look for association between various factors and surgical outcome. Factors found to have significant associations were then analyzed using multivariate regression model to determine whether they can be considered as independent factors. A value of p<0.05 was considered significant.
Results
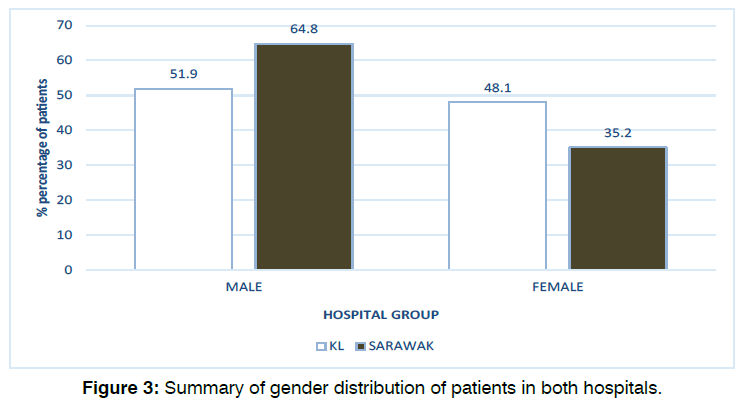
A total of 187 babies in KL fulfilled the study inclusion criteria between January 2014 and December 2019 inclusive. The median age of patients at the time of their first (screening) test was 40 days old (mean: 46.8 ± 30) and the median age of patients at the time of their second (diagnostic) test was 150 days old (mean: 145.2 ± 71). Our data also found that 51.9% (97) of the infants were male and 48.1% (90) were female.
193 babies in Sarawak fulfilled the study inclusion criteria between January 2014 and December 2019 inclusive. The median age of patients at the time of their first (screening) test was 25 days old (mean: 25 ± 26, range: 1-180) and the median age of patients at the time of their second (diagnostic) test was 60 days old (mean: 81.2 ± 44, range: 25-360). Our data also found that 64.8% (125) of the infants were male and 35.2% (68) were female. Figure 2 and Figure 3 illustrates the age and gender distribution in each hearing test groups in both hospitals. There were statistically significant differences in patient’s age between both hospitals for both the first (screening) test (p<0.001) and second (diagnostic) test (p<0.001). There was also statistically significant difference in terms of gender distribution between the two hospitals (p=0.011).
Figure 3: Summary of gender distribution of patients in both hospitals.
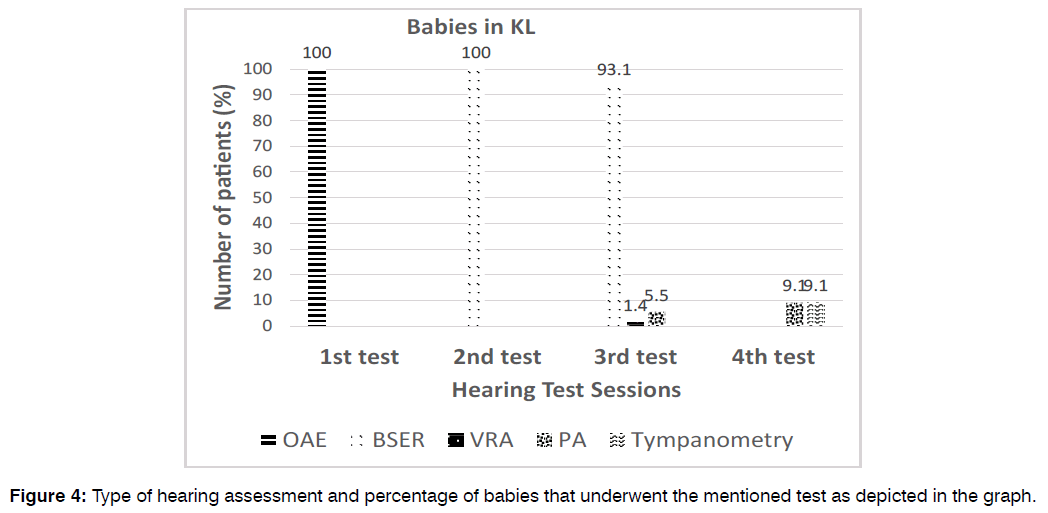
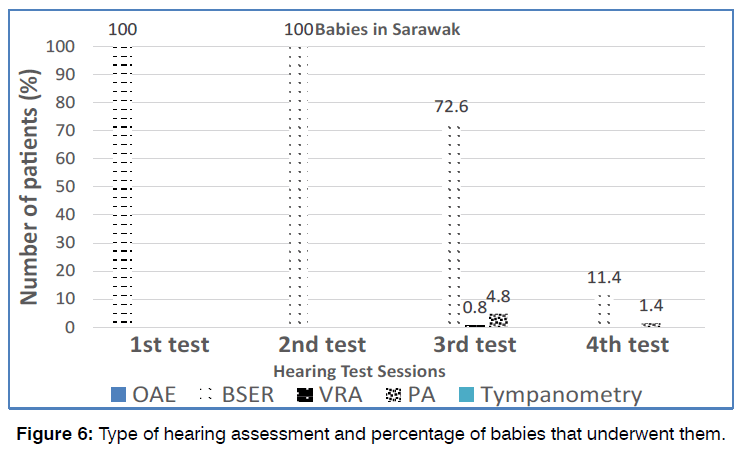
Hearing Tests and Management Characteristics: All 187 KL babies (100%) underwent the first (screening) test and second (diagnostic) test, however only 73 (39%) babies underwent the third and 11 (5.9%) patients underwent the fourth audiological tests. All babies (100%) received OAE in the first screening test and BSER in the subsequent diagnostic test. On the other hand, for the third hearing tests, only 93.1% of patients underwent BSER while the others underwent play audiometry (5.5%) and VRA (1.4%). For the fourth hearing tests, majority of babies (81.8%) received BSER test, while the others received either play audiometry (9.1%) or tympanometry (9.1) illustrated in Figure 4.
Figure 4: Type of hearing assessment and percentage of babies that underwent the mentioned test as depicted in the graph.
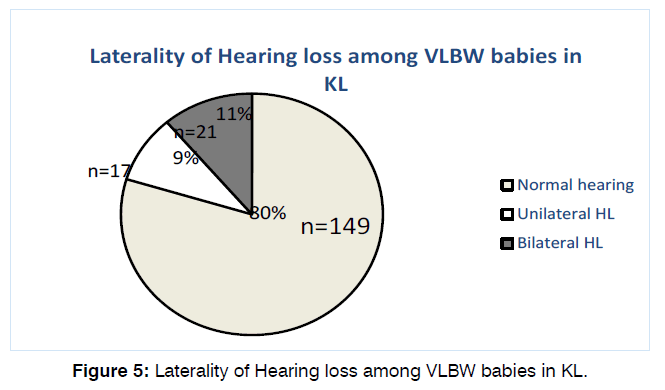
79.7% (149) of the babies have normal hearing tests on both OAE and BSER in both ears. Of the remaining 38 patients, 17 (9.1%) had unilateral and 21 (11.2%) had bilateral hearing loss as shown in Figure 5.
Figure 5: Laterality of Hearing loss among VLBW babies in KL.
Majority of the babies with hearing loss were found to be male (52.6%) and occurs at more than 33 weeks of gestational age wga (44.7%). Most babies presented with bilateral SNHL (21.1%) at >33 wga followed by bilateral SNHL at 29-32 wga. The detailed breakdown of the type and degree of hearing loss with associated gestational age is illustrated in Table 1.
| Weeks in gestation(wga) | Hearing Level |
<28 wga (n=6) | 29-32 wga (n=15) | >33 wga (n=17) | |||
|---|---|---|---|---|---|---|---|
| Unilateral | Bilateral | Unilateral | Bilateral | Unilateral | Bilateral | ||
| SNHL | 20-40dB | 0 | 0 | 1 | 4 | 3 | 4 |
| 41-70dB | 1 | 0 | 1 | 1 | 0 | 2 | |
| 71-90dB | 0 | 0 | 0 | 0 | 0 | 1 | |
| >90dB | 0 | 0 | 0 | 0 | 0 | 1 | |
| Total | 1 (2.6%) | 0 | 2 (5.3%) | 5(13.2%) | 3 (7.9%) | 8(21.1%) | |
| CHL | 20-40dB | 3 | 1 | 2 | 2 | 2 | 1 |
| 41-70dB | 1 | 0 | 2 | 1 | 0 | 1 | |
| 71-90dB | 0 | 0 | 0 | 0 | 0 | 0 | |
| >90dB | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total | 4(10.5%) | 1(2.6%) | 4(10.5%) | 3 (7.9%) | 2 (5.3%) | 2 (5.3%) | |
| MHL | 20-40dB | 0 | 0 | 0 | 0 | 0 | 0 |
| 41-70dB | 0 | 0 | 0 | 0 | 0 | 0 | |
| 71-90dB | 0 | 0 | 0 | 1 | 1 | 1 | |
| >90dB | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total | 0 | 0 | 0 | 1 (2.6%) | 1 (2.6%) | 1 (2.6%) | |
Table 1: Prevalence of different types and degree of hearing loss in relation to the gestational age for patients at HCTM.
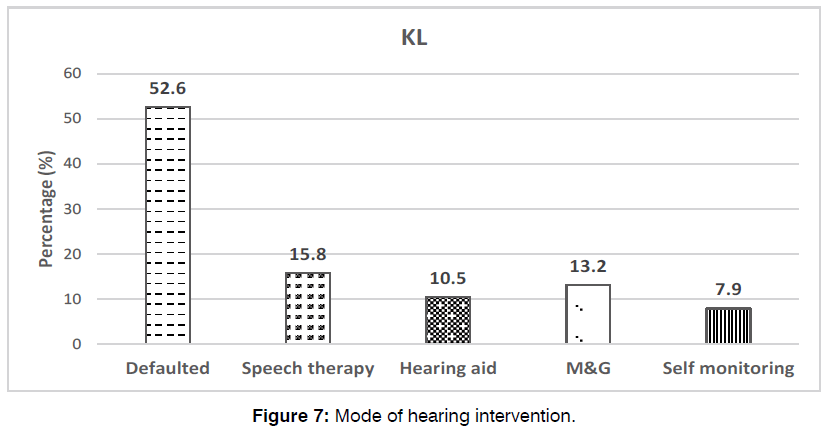
The median age of these babies with hearing impairment receiving intervention was 583 days old (19 months old) (mean: 591.6 ± 343). Unfortunately, approximately half (52.6%) of them were loss to follow-ups. Of the remaining, majority (15.8%) received speech therapy, while the rest had hearing aids (10.5%), myringotomy and grommet insertions (13.2%) and self-monitoring (7.9%) depicted in Figure 6.
Figure 6: Type of hearing assessment and percentage of babies that underwent them.
193 babies in Sarawak (100%) underwent the first (screening) test and second (diagnostic) test, whereas only 151 (78.2%) underwent the third and 22 (11.4%) babies underwent the fourth audiological tests. All babies (100%) received OAE in the first screening test and BSER in the subsequent second, third and fourth diagnostic test. This is illustrated in Figure 7.
Figure 7: Mode of hearing intervention.
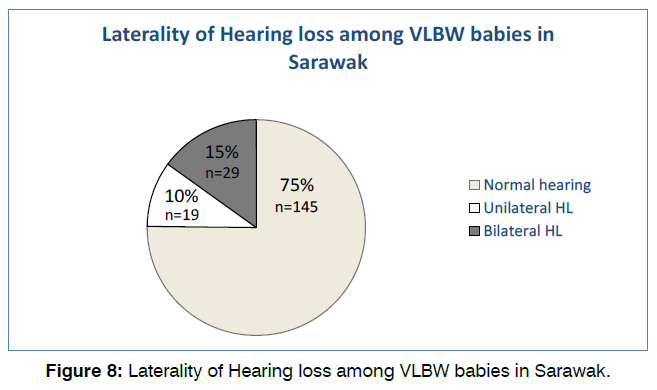
75.1% (145) of the babies have normal hearing tests on both OAE and BSER in both ears. Of the remaining 48 patients, 19 (9.8%) had unilateral and 29 (15%) had bilateral hearing loss as shown in Figure 8. Majority of babies with hearing loss were found to be male (56.3%) and occurs at more than 33 weeks of gestational age (wga) (56.3%). Most babies presented with unilateral CHL (14.6%) at >33 wga followed by mild bilateral SNHL at 29-32 wga and more than 33 wga. No babies in this population presented with mixed hearing loss.
Figure 8: Laterality of Hearing loss among VLBW babies in Sarawak.
The detailed breakdown of the types and degree of hearing loss with associated gestational age was illustrated in Table 2.
| Weeks in gestation (wga) | Hearing level (dB) | <28 wga (n=7) | 29-32 wga (n=14) | >33 wga (n=27) | |||
|---|---|---|---|---|---|---|---|
| Unilateral | Bilateral | Unilateral | Bilateral | Unilateral | Bilateral | ||
| SNHL | 20-40dB | 1 | 0 | 0 | 2 | 1 | 2 |
| 41-70dB | 0 | 0 | 0 | 1 | 1 | 0 | |
| 71-90dB | 0 | 1 | 0 | 0 | 1 | 3 | |
| >90dB | 1 | 1 | 0 | 2 | 2 | 2 | |
| Total | 2 (4.2%) | 2 (4.2%) | 0 | 5(10.4%) | 5 (10.4%) | 7(14.6%) | |
| CHL | 20-40dB | 2 | 1 | 3 | 6 | 7 | 6 |
| 41-70dB | 0 | 0 | 0 | 0 | 0 | 2 | |
| 71-90dB | 0 | 0 | 0 | 0 | 0 | 0 | |
| >90dB | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total | 2 (4.2%) | 1 (2.1%) | 3 (6.3%) | 6(12.5%) | 7 (14.6%) | 8(16.7%) | |
Table 2: Prevalence of different types and degree of hearing loss in relation to the gestational age for patients at Sarawak.
The median age of babies with hearing impairment receiving intervention was 815 days old (27 months old) (mean: 410.6 ± 189, range: 70–900). 32.1% of patients were loss to follow-ups. Of the remaining babies, majority (37.5%) had myringotomy and grommet insertions, while the rest had hearing aids (18.8%), self-monitoring (10.4%), speech therapy (6.25%), cochlear implant (4.2%) and auditory brainstem implant (2.1%).
Risk Factors for Hearing Loss: Majority of the babies in KL were found to have risk factors of low birth weight (<1500g) (81.8%), gestational age between 29 to 32 weeks (49.2%), neonatal jaundice (80.6%), RDS (61%), spending more than 5 days in NICU (62.6%) and receiving ototoxic drugs (56.7%).
On the other hand, babies who were diagnosed with hearing loss were found to have risk factors of low birth weight (<1500g) (16.6%), gestational age more than 33 weeks (9.1%), neonatal jaundice (14.5%), RDS (11.8%), spending more than 5 days in NICU (13.9%) and receiving ototoxic drugs (10.7%). Table 3 illustrates the detailed breakdown of all the risk factors associated with hearing loss in this study population.
| Risk factors | SNHL n (%) | CHL n (%) | MHL n (%) | P-value |
|---|---|---|---|---|
| Gender Male Female |
11 (5.9) 8 (4.3) |
9 (4.8) 7 (3.7) |
0 3 (1.6) |
0.304 |
| Birth weight <1500g <1000g |
16 (8.6) 3 (1.6) |
12 (6.4) 4 (2.1) |
3 (1.6) 0 |
0.743 |
| Gestational age (weeks) <28 29-32 >33 |
1 (0.5) 7 (3.7) 11 (5.9) |
5 (2.7) 7 (3.7) 4 (2.1) |
0 1 (0.5) 2 (1.1) |
0.003* |
| Neonatal jaundice Yes No |
10 (5.4) 9 (4.8) |
16 (8.6) 0 |
1 (0.5) 2 (1.1) |
<0.001* |
| RDS Yes No |
11 (5.9) 8 (4.3) |
9 (4.8) 7 (3.7) |
2 (1.1) 1 (0.5) |
0.959 |
| NICU >5 days Yes No |
14 (7.5) 5 (2.7) |
9 (4.8) 7 (3.7) |
3 (1.6) 0 |
0.360 |
| Ototoxic drugs Yes No |
10 (5.4) 9 (4.8) |
7 (3.7) 9 (4.8) |
3 (1.6) 0 |
0.311 |
| IVH Yes No |
6 (3.2) 13 (7) |
3 (1.6) 13 (7) |
2 (1.1) 1 (0.5) |
0.403 |
| Mechanical ventilation Yes No |
7 (3.7) 12 (6.4) |
3 (1.6) 13 (7) |
1 (0.5) 2 (1.1) |
0.639 |
| IUGR Yes No |
4 (2.1) 15 (8) |
3 (1.6) 13 (7) |
0 3 (1.6) |
0.859 |
| G6PD Yes No |
2 (1.1) 17 (9.1) |
0 16 (8.6) |
0 3 (1.6) |
0.064 |
| Syndromic/dysmorphism Yes No |
5 (2.7) 14 (7.5) |
1 (0.5) 15 (8) |
2 (1.1) 1 (0.5) |
<0.001* |
| Low APGAR Yes No |
7 (3.7) 12 (6.4) |
3 (1.6) 13 (7) |
0 3 (1.6) |
0.411 |
| Positive family history Yes No |
1 (0.5) 18 (9.6) |
0 16 (8.6) |
0 3 (1.6) |
0.031* |
Table 3: Univariate analysis of various risk factors and diagnosis of hearing loss for babies in KL. * Indicates p<0.05.
Associations between Risk Factors and Hearing Loss: Univariate analysis of various risk factors revealed statistically significant associations between gestational age (week) (p=0.003), presence of neonatal jaundice (p<0.001), syndromes or dysmorphism (p=<0.001) and positive family history (p=0.031) with diagnosis hearing impairment. There were no statistically significant associations between gender, birthweight, RDS, spending more than 5 days in NICU, ototoxic drugs, IVH, mechanical ventilation, IUGR, G6PD and low APGAR scores with prevalence of hearing loss (Table 3).
When analyzed for independent factors with multivariate regression analysis, only gestational age (week) (p=0.044) and presence of syndromes or dysmorphism (p<0.001) were found to have statistically significant association with prevalence of hearing loss in this group of patients (Table 4).
| Independent variable | Unstandardized coefficient (β) | 95% CI | P value |
|---|---|---|---|
| Gestational age (weeks) | 0.143 | 0.004 to 0.281 | 0.044* |
| Neonatal jaundice Syndromic/dysmorphism Positive family history |
0.046 -0.896 0.208 |
-0.205 to 0.297 -1.323 to -0.468 -1.182 to 1.599 |
0.720 <0.001* 0.768 |
Table 4: Results of multivariate regression analysis with unstandardized coefficient (β), 95% CI and p-value for babies in KL.
Characteristics of Risk Factors: Majority of babies in Sarawak study were found to have risk factors of low birth weight (<1500g) (95.9%), gestational age more than 33 weeks (54.9%), neonatal jaundice (62.7%), spending more than 5 days in NICU (65.3%) and receiving ototoxic drugs (60.4%).
On the other hand, babies who were diagnosed with hearing loss were found to have risk factors of low birth weight (<1500g) (23.3%), gestational age more than 33 weeks (14%), neonatal jaundice (17.1%), RDS (13.5%), spending more than 5 days in NICU (17.1%) and receiving ototoxic drugs (16.1%). Table 5 illustrates the detailed breakdown of all the risk factors associated with hearing loss in this study population.
| Risk factors | SNHL n (%) | CHL n (%) | p-value |
|---|---|---|---|
| Gender Male Female |
10 (5.2) 11 (5.7) |
17 (8.8) 10 (5.2) |
0.197 |
| Birth weight <1500g <1000g |
19 (9.8) 2 (1) |
26 (13.5) 1 (0.5) |
0.423 |
| Gestational age (weeks) <28 29-32 >33 |
4 (2.1) 5 (2.6) 12 (6.2) |
3 (1.6) 9 (4.7) 15 (7.8) |
0.918 |
| Neonatal jaundice Yes No |
13 (6.7) 8 (4.1) |
20 (10.4) 7 (3.6) |
0.417 |
| RDS Yes No |
11 (5.7) 10 (5.2) |
11 (5.7) 16 (8.3) |
0.709 |
| NICU >5 days Yes No |
17 (8.8) 4 (2.1) |
16 (8.3) 11 (5.7) |
0.248 |
| Ototoxic drugs Yes No |
17 (8.8) 4 (2.1) |
14 (7.3) 13 (6.8) |
0.098 |
| IVH Yes No |
10 (5.2) 11 (5.7) |
6 (3.1) 20 (10.4) |
0.032* |
| Mechanical ventilation Yes No |
10 (5.2) 11 (5.7) |
11 (5.7) 16 (8.3) |
0.099 |
| IUGR Yes No |
1 (0.5) 20 (10.4) |
2 (1) 25 (13) |
0.851 |
| G6PD Yes No |
0 21 (10.9) |
0 27 (14) |
1.000 |
| Syndromic/dysmorphism Yes No |
5 (2.6) 16 (8.3) |
7 (3.6) 20 (10.4) |
0.010* |
| Low APGAR Yes No |
10 (5.2) 11 (5.7) |
8 (4.1) 18 (9.4) |
0.174 |
| Positive family history Yes No |
1 (0.5) 20 (10.4) |
0 27 (14) |
0.016* |
Table 5: Univariate analysis of various risk factors and diagnosis of hearing loss for patients in Sarawak. * Indicates p<0.05.
Associations between Risk Factors and Hearing Loss: Univariate analysis of various risk factors revealed statistically significant associations between presence of IVH (p=0.032), syndromes or dysmorphism (p=0.010) and positive family history (p=0.016) with diagnosis hearing impairment. There was no statistically significant association between gender, birthweight, gestational age, neonatal jaundice, RDS, spending more than 5 days in NICU, ototoxic drugs, mechanical ventilation, IUGR, G6PD and low APGAR scores with prevalence of hearing loss (Table 5).
When analyzed for independent factors with multivariate regression analysis, only presence of syndromes or dysmorphism (p<0.001) were found to have statistically significant association with prevalence of hearing loss in this group of patients (Table 6).
| Independent variable | Unstandardized coefficient (β) | 95% CI | P value |
|---|---|---|---|
| IVH Syndromic/dysmorphism Positive family history |
0.099 -0.964 0.160 |
-0.113 to 0.311 -1.397 to -0.530 -1.225 to 1.545 |
0.359 <0.001* 0.819 |
Table 6: Results of multivariate regression analysis with unstandardized coefficient (β), 95% CI and p-value for patients in Sarawak.
Discussion
The goal of early detection and intervention for hearing loss among at risk infants cannot be emphasized enough to gain literacy competence among this babies. In this study conducted in two tertiary care hospitals, the results that were analyzed from KL showed out of the 187 babies, 6 (15.8%) had unilateral Sensorineural Hearing Loss (SNHL) and 13 (34.3% ) had bilateral SNHL and in Sarawak out of 193 babies, the percentage with bilateral SNHL was 14.6%(n=7) and unilateral SNHL 4.2%(n=2).Most babies in KL that had bilateral SNHL were born at a gestational age of more than 33 weeks (21.1%), followed by at 29- 32 weeks of gestation (13.2%).Similar was found among Sarawak babies ,where 54.9% were born at 33 weeks of gestation. It is reported in literature, where studies have shown that premature children in comparison to babies born full term had a higher prevalence of hearing loss [5-8]. It is found that prematurity alone may not significantly impact the outcome of hearing, however in the presence of other risk factors; it may have synergistic influence on the hearing outcome.
In a study by Silvia et al [9], among 364 premature babies with birthweight less than 1500 grams, 36.4% were reported to have SNHL. Here it was reported that the hearing loss were reported among babies that were born at a gestational age less than 32 weeks. Katarzyna et al, reported 11% of hearing loss among children born less than 25 weeks of gestation, and were associated at least with one co-existing risk factor.
In term of conductive hearing loss 42.1 % of KL babies and 56.4 % in Sarawak was reported. This is comparable with a study by Maria et al, among 929 live newborns, where they reported 62.5% conductive hearing loss among VLBW babies. The contributing factors to the development of conductive loss arises from structural immaturity of the Eustachian tube compounded by naïve immune system, which increases the risk of developing auditory tube dysfunction, inflammation of the middle ear mucosa and subsequently middle ear effusion as a sequalae [10]. Children with mild CHL were also often discharged from screening programs with home surveillance, showed delayed hearing loss that goes undiagnosed [11]. This high reporting negatively impacts hearing and language development of the affected child, and may even progress to a permanent hearing loss if not intervened. This further carries a big impact on the child’s speech and future academic performances however mild the hearing loss is [12].
In our study, neonatal jaundice, prolonged admission in the NICU ototoxicity and respiratory distress syndrome were the commonly co-existing risk factor among babies born with birth weight less than 1500 gram in both hospitals. However, in both groups, when analyzed for independent risk factor, presence of syndromes or dysmorphism (p<0.001) was the only risk factor to have statistically significant association with hearing loss and hyperbilirubinemia was not one of the cause. This similar outcome was also reported by Katrzyna et al, in a Polish UNHS among preterm babies born less than 33 weeks’ gestation, from 2010 to 2013 [13]. This is likely contributed to the efficient initiation of phototherapy in both of our centers to avoid catastrophic rise in the bilirubin level. Hyperbilirubinemia is known to cause damage to the auditory nuclei, the auditory nerve and spiral ganglion, by interfering with intracellular neuronal calcium homeostasis, and this is further modulated by other risk factors. De Vries et al [14],reported children born preterm with high bilirubin levels and weighing less than 1500grams carried higher risk if hearing impairment compared to their health counterparts weighing more than 1500grams [9].
Despite not being frequently reported in our study, both hospitals showed dysmorphism and syndromic children to significantly contribute to hearing impairment. Craniofacial anomalies including cleft palate and canal abnormalities were seen in both groups. It is also interesting to note that intraventricular haemorrhage was seen to be associated with diagnosis of hearing impairment in the Sarawak group. This is comparable with a study done by Da Silva et al, where significant delay in absolute latency values and interpeak intervals on Brainstem Evoked Response (BSER) among children with peri-intraventricular hemorrhage [15].The study involved only infants with normal otoacoustic emissions, which then demonstrated synaptic dysfunction from the auditory pathway to the brainstem among babies with intraventricular hemorrhage. These babies shared similar risk factors of birth weight <1500grams, prolonged ICU stay, low APGAR score and use of ototoxic medication. There was no significant correlation between the above factors and alterations in the BSER [15].Another report by Angrisani et al, the auditory central alteration, characterized by increased latency of wave III and V was higher among preterm infants with intraventricular hemorrhage [16].
Another important cause, which is hypoxia with Respiratory Distress Syndrome (RDS), has been reported to be associated with hearing impairment in premature infants. In our study, both hospitals reported hearing loss among infants requiring mechanical ventilation and prolonged oxygen supplementation for RDS. In the KL group 10 % of mechanically ventilated babies had associated SNHL, whereas in the Sarawak it reported 7% among the patients. This is likely attributed to an early noise-induced hearing loss contributed by the exposure of constant background noise of the ventilator. It is found that neonatal stereocilia are more easily over-stimulated. The initial sign would be damage to the outer hair cell [12].A study among temporal bones of preterm by Amatuzzi et al, reported 27% showed selective loss of inner ear hair cells among mechanically ventilated babies [17].One study more than 50 % of infants surviving severe respiratory failure developed delayed SNHL at 4 years of age [18]. The physiology of this delayed onset is still unclear; however it is likely due to degeneration points along the auditory pathway. Another indicator for perinatal hypoxia are infants that had low APGAR scores at 5 and 10 minutes, however was not statistically significant in our study p=0.174 and p=0.411 for KL and Sarawak respectively. Hypoxia affects the spiral ganglion first and may cause irreversible damage to the outer hair cells.
Exposure to aminoglycoside is a risk factor for hearing loss among infants receiving them prophylactically or as an empiric treatment for serious bacterial infection in the NICU. 60.4% of infants in KL and 56.7% infants in Sarawak group were given ototoxic medication, out of which 16.1% was linked to hearing impairment in KL group and 10.7% in the Sarawak group. This is found to be higher than reported in literature. A recent study in Switzerland in 2016 by Alline Fuchs et al, 76% of infants was treated with Gentamicin, and the incidence of SNHL was 1.58% among their very low birth weight infant population [19]. However another study reported by Wang et al, infants receiving aminoglycosides, 11% were reported to have hearing impairment. This discrepancy may be attributed by different dosing adjustments, duration of treatment, as well as genetic susceptibility. It has been found that mitochondrial DNA mutations have been linked to sensitivity to aminoglycoside, however screening for these genetic variants is difficult as aminoglycosides are often administered at first day of life, when diagnosis of presumed sepsis is made [20, 21]. Genetic study was not done and is beyond the scope of our study. Ototoxicity also may be potentiated with host induced inflammatory response to on-going infection as lysed bacteria release immunogens that itself carries risks of ototoxicity [22].
Infants that are premature with very low birth weight have been shown in this study to have increased risk of hearing loss, hence early activation of habilitation can promote maturation of the auditory pathway. Studies have shown that hearing loss can developed in a delayed manner, especially among high risk neonates, that may have a normal diagnostic hearing assessment in the early stages, however show threshold changes within 6 months to 12 months of age [23, 24]. The mean age receiving intervention range from 19 months to 27th month of age from both hospitals. This unfortunately does not fulfill the recommended time and standard of care suggested by the Joint Community of Infant Hearing that requires early intervention within 6 months of age. 29.3% of the children with hearing loss from both hospitals received hearing aid and 4.2% were implanted with cochlear implant.
The choice of intervention depends on the type, the degree and the cause of hearing loss. Otitis media with effusion was treated and reversed by surgical intervention. In both of our hospitals, 50.7% of babies eventually underwent myringotomy and grommet insertion for either unilateral CHL or bilateral CHL, where the hearing levels were between 20 to 40dB. Often children with CHL are discharged from screening programs with home surveillance, which results with a delayed permanent hearing loss that goes undiagnosed [23]. In our study, 22.05% of children were referred for speech therapy with delayed speech and language acquisition. This shows that the insult from auditory deprivation among these babies affects intellectual, cognitive and social development. The NIH in 2015 reported among 870 children, about 28% of babies very low birthweight required speech intervention for poor language acquisition [24, 25]. Comparing our results with the literature, very low birth weight with hyperbilirubinemia, neonatal hypoxia, DS and NICU stay more than 5 days were significantly associated with hearing impairment, unlike our findings where we found significant relationship between very low birth weight hearing loss and the risk factors: dysmorphism, syndromic and exposure to aminoglycosides. Our study may be under reported because none of the babies in the study group with hyperbilirubinemia required exchange transfusion, may not reflect the true outcome of hearing.
Conclusion
The percentage of hearing loss among children with VLBW and prematurity was 20.3% among the KL babies and 24.8% among the Sarawak babies, which is a comparable available report in literature. Gestational age (p=0.044) and presence of dysmorphism (p<0.001) were found to have statistically significant association with prevalence of hearing loss in our study population. The rate of failed diagnostic screenings was also high among VLBW as they experience higher rate of middle ear effusion causing conductive hearing loss.
References
- Dalzell L, Orlando M, MacDonald M, Berg A, Bradley M, Cacace A, et al. The New York State universal newborn hearing screening demonstration project: ages of hearing loss identification, hearing aid fitting, and enrollment in early intervention. Ear Hear. 2000;21(2):118-30.
- Lorenz JM. Survival of the extremely preterm infant in North America in the 1990s. Clin Perinatol. 2000;27(2):255-62.
- Joint Committee on Infant Hearing. Year 2007 position statement: Principles and guidelines for early hearing detection and intervention programs. Pediatrics. 2007;120(4):898-921.
- Borkoski-Barreiro SA, Falcón-González JC, Liminana-Canal JM, Ramos-Macías Á. Evaluation of very low birth weight (= 1500 g) as a risk indicator for sensorineural hearing loss. Acta Otorrinolaringol Esp. 2013;64(6):403-8.
- Bielecki I, Horbulewicz A, Wolan T. Risk factors associated with hearing loss in infants: an analysis of 5282 referred neonates. Int J Pediatr Otorhinolaryngol. 2011;75(7):925-30.
- Tiensoli LO, Goulart LM, Resende LM, Colosimo EA. Hearing screening in a public hospital in Belo Horizonte, Minas Gerais, Brazil: hearing impairment and its risk factors in neonates and infants. Public Health Notebooks. 2007;23(6):1431-41.
- Cañete O, Torrente M. Evaluation of the early detection program for hearing loss in extremely preterm newborns (RNPE), experience Hospital Padre Hurtado. J Otolaryngol and Head and Neck Surg. 2011;71(2):117-22.
- Martines F, Salvago P, Bentivegna D, Bartolone A, Dispenza F, Martines E. Audiologic profile of infants at risk: experience of a Western Sicily tertiary care centre. Int J Pediatr Otorhinolaryngol. 2012;76(9):1285-91.
- Borkoski-Barreiro SA, Falcón-González JC, Liminana-Canal JM, Ramos-Macías Á. Evaluation of very low birth weight (= 1500 g) as a risk indicator for sensorineural hearing loss. Acta Otorrinolaringol Esp. 2013;64(6):403-8.
- Colella-Santos MF, Hein TA, de Souza GL, do Amaral MI, Casali RL. Newborn hearing screening and early diagnostic in the NICU. Biomed Res Int. 2014.
- Collins A, Beswick R, Driscoll C, Kei J. Conductive Hearing Loss within Universal Newborn Hearing Screening Programs: A Systematic Review. J Hear Sci. 2019;9(3).
- Borges LR, Paschoal JR, Colella-Santos MF. (Central) Auditory Processing: the impact of otitis media. Clinics. 2013;68:954-9.
- Wroblewska-Seniuk K, Greczka G, Dabrowski P, Szyfter-Harris J, Mazela J. Hearing impairment in premature newborns—Analysis based on the national hearing screening database in Poland. PloS One. 2017;12(9):e0184359.
- De Vries LS, Lary S, Whitelaw AG, Dubowitz LM. Relationship of serum bilirubin levels and hearing impairment in newborn infants. Early Hum Dev. 1987;15(5):269-77.
- Da Silva LS, Ribeiro GE, Montovani JC, da Silva DP. The effect of peri-intraventricular hemorrhage on the auditory pathway of infants. Int J Pediatr Otorhinolaryngol. 2018;112:24-6.
- Angrisani RG, Fagá AM, Goulart AL, Azevedo MF. Intracranial hemorrhage and central auditory disorders in neonates. Revista CEFAC. 2016;18:341-7.
- Amatuzzi M, Liberman MC, Northrop C. Selective inner hair cell loss in prematurity: a temporal bone study of infants from a neonatal intensive care unit. J Assoc Res Otolaryngol. 2011;12:595-604.
- Robertson CM, Tyebkhan JM, Hagler ME, Cheung PY, Peliowski A, Etches PC. Late-onset, progressive sensorineural hearing loss after severe neonatal respiratory failure. Otol Neurotol. 2002;23(3):353-6.
- Fuchs A, Zimmermann L, Bickle Graz M, Cherpillod J, Tolsa JF, Buclin T, et al. Gentamicin exposure and sensorineural hearing loss in preterm infants. PloS One. 2016;11(7):e0158806.
- Ealy M, Lynch KA, Meyer NC, Smith RJ. The prevalence of mitochondrial mutations associated with aminoglycoside-induced sensorineural hearing loss in an NICU population. Laryngoscope. 2011;121(6):1184-6.
- Bitner-Glindzicz M, Rahman S, Chant K, Marlow N. Gentamicin, genetic variation and deafness in preterm children. BMC Pediatr. 2014;14(1):1-4.
- Koo JW, Quintanilla-Dieck L, Jiang M, Liu J, Urdang ZD, Allensworth JJ, et al. Endotoxemia-mediated inflammation potentiates aminoglycoside-induced ototoxicity. Sci Transl Med. 2015;7(298):298.
- Kral A, O'Donoghue GM. Profound deafness in childhood. N Engl J Med. 2010;363(15):1438-50.
- Aimoni C, Crema L, Savini S, Negossi L, Rosignoli M, Sacchetto L, et al. Hearing threshold estimation by Auditory Steady State Responses (ASSR) in children. Acta Otorhinolaryngol Ital. 2018;38(4):361.
- Low birth weight linked to communication problems in children. The National Institute on Deafness and Other Communication Disorders, a member of the U.S. National Institutes of Health. 2019.
1Department of Otorhinolaryngology, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia
2Department of Otorhinolaryngology, Sarawak General Hospital, Kuching Sarawak, Malaysia
3Department of Rehabilitation Services, Uniiversiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia
Send correspondence to:
Dr. Asma Abdullah
Department of Otorhinolaryngology, Department of Otorhinolaryngology, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia, Tel no: 600391456599, Email: asmappukm@gmail.com
Paper submitted on February 24, 2022; and Accepted on March 02, 2023
Citation: Abdullah A, Wilfred R, Mohamad Yusof AN, Wan Hashim WF. Prevalence of Hearing Loss among Very Low Birth Weight (VLBW) Babies in Two Tertiary Hospitals in Malaysia. Int Tinnitus J. 2023;27(1):16-26.