The International Tinnitus Journal
Official Journal of the Neurootological and Equilibriometric Society
Official Journal of the Brazil Federal District Otorhinolaryngologist Society
ISSN: 0946-5448

Google scholar citation report
Citations : 12717
The International Tinnitus Journal received 12717 citations as per google scholar report
The International Tinnitus Journal peer review process verified at publons
Indexed In
- Excerpta Medica
- Scimago
- SCOPUS
- Publons
- EMBASE
- Google Scholar
- Euro Pub
- CAS Source Index (CASSI)
- Index Medicus
- Medline
- PubMed
- UGC
- EBSCO
Volume 26, Issue 1 / June 2022
Review Article Pages:27-41
10.5935/0946-5448.20220005
The Role of Gut Dysbiosis in the Pathophysiology of Tinnitus: A Literature Review
Authors: Imam Megantara, Gisela Liani Wikargana,Yussy Afriani Dewi, Agung Dinasti Permana, Nova Sylviana
PDF
Abstract
Introduction: For years, tinnitus has become a prevalent symptom in the ENT department. However, the mechanism underlying tinnitus remains unclear. There is increasing evidence that tinnitus is related to an abnormal central gain in the central auditory system and increased neuroinflammatory mediators. On the other hand, recent studies have shown that gut dysbiosis plays a crucial role in brain function, and gut dysbiosis contributes to several neurological diseases. Hence, giving insight into its possible involvement in tinnitus.
Aim: This study evaluated the potential role of gut microbiota dysbiosis in the path mechanism of tinnitus, mainly through its effect on neurotransmitter production and neuroinflammation induction.
Methods: This study uses a literature review approach, inclusive only of experimental studies in the recent five years discussing gut dysbiosis, neurotransmitter, neuroinflammation, and tinnitus signaling. Results: From 22 relevant literature, we found that gut dysbiosis impacts neurotransmitter production such as GABA and 5-HT and contributes to the neuroinflammatory process by increasing pro-inflammatory cytokines and activated microglia. These altered neurotransmitter profiles and triggered pro-inflammatory mediators were also found in tinnitus.
Conclusion: Gut microbiota dysbiosis is likely to underlie tinnitus’s pathomechanism by altering neurotransmitter production and activating the neuroinflammatory response in the brain.
Keywords: Gut-Brain Axis, Gut Dysbiosis, Neuroinflammation, Neurotransmitter, Tinnitus Signaling.
Introduction
Tinnitus is defined as an auditory perception or sensation without any external stimuli. Tinnitus itself originates from the Latin word, “tinnire” which means “to ring” [1,2]. Patients with tinnitus often described the auditory perception as ringing, buzzing, hissing, or whistling [1]. About 15-20% of people in the world will experience tinnitus in their lifetime, and constant noise perception in the ears can range from mild to very debilitating [1,2]. Tinnitus can also affect children and adolescents with 7.5%-60% prevalence [3]. In children with normal hearing, the majority is around 6%-36%, while in deaf children, approximately 55% [4].
Tinnitus can negatively impact the patients’ psychological and emotional well-being, lowering their quality of life [5]. Some of the most common psychological impacts experienced by tinnitus patients are concentration disturbance, sleep disorder, and mood disorder, which lead to unstable emotions such as getting angry more easily. Patients with tinnitus also have a greater risk of experiencing depression, anxiety, and insomnia, thus affecting their quality of life [5,6].
The etiology of tinnitus is not fully known and is often multifactorial.7 Most of the cases are related to hearing impairment and changes in the inner ear [7,8]. Previous studies showed that one of the significant risk factors for tinnitus is excessive noise exposure. The exposure could be both long-term or a brief loud noise that can result in some changes in the structures of the inner ear. Studies also showed that the prevalence of tinnitus with hearing loss or hearing impairment is higher than those with normal hearing [4,9]. In addition, neurological disorders or head trauma, such as skull fractures, temporomandibular joint disorders, whiplash injuries, and multiple sclerosis, as well as other conditions, such as thyroid disease, hyperlipidemia, and vascular disease, including arterial bruit and arteriovenous malformation can also be related to tinnitus, which contributes to 5-10% of tinnitus cases [7].
The mechanism underlying tinnitus is also not fully understood [10]. Some experts have proposed the central gain control theory, suggesting that tinnitus is related to neural plasticity, an increase in spontaneous neural activity due to loss or reduction of sensory input. Evidence of an increase in auditory neural activity, particularly in fusiform cells, inferior colliculus (IC), and medial geniculate body (MGB), also reorganization of the tonotopic map in the auditory cortex of animals showing evidence of behavioral tinnitus reinforces the theory [11-13]. In their study reported a significant reduction in gamma-aminobutyric acid (GABA), the major inhibitory neurotransmitter in the brain, in the auditory cortex of patients with tinnitus. This loss of inhibition is likely associated with increased neuronal activity in tinnitus [11,14].
Besides the central gain control theory, recent evidence suggests that neuroinflammation also underlies tinnitus. Wang et al. indicate that inflammation of the primary auditory pathway induced by noise-induced hearing loss (NIHL) plays a role in developing tinnitus in mice. Their research showed that mice with NIHL had elevated proinflammatory cytokines and activated microglia, a non-neuronal cell in the primary auditory cortex, the two key features of neuroinflammatory responses. Proinflammatory cytokine such as TNF-α is required for noise-induced neuroinflammation and tinnitus. Experimental rats no longer showed signs of tinnitus after being given TNF-α inhibitors. Furthermore, genetic knockout TNF-α prevented neuroinflammation and ameliorated the behavioral phenotype associated with tinnitus in mice with NIHL [15].
In recent years, the gut microbiome has attracted the attention of researchers. Various studies have shown that microbiota has specific and complex cross-talk with their hosts, particularly with the central nervous system (CNS), creating the “gut-brain axis”. The secretion of various metabolites, bacterial structural components, and signaling molecules were involved in this gut, gut microbiota, and brain’s communication. In addition, imbalances in the gut microbiota composition can modulate the immune system and barrier function of tissues such as the blood-brain barrier (BBB), so impaired gut-brain interactions may give rise to various neurological disturbances [16,17]. Interestingly, recent studies have shown that the gut microbiota can regulate the production of neurotransmitters, such as GABA and serotonin [17-19], and inflammatory mediators, such as TNF-α and IL-6 [20,21]. Furthermore, this altered regulation of gut microbiota could contribute to the changes in the concentration of neurotransmitters and inflammatory mediators which possibly related to tinnitus [15,16,22,23].
So far, tinnitus is still an unsolved clinical problem. There is no cure for tinnitus. Some treatments may help some people, but none work for everyone. Increased knowledge about the pathomechanism of tinnitus can improve tinnitus management. However, recent studies indicating altered neurotransmitter production and activity and increased levels of proinflammatory cytokines in experimental animals exhibiting tinnitus behavior have provided insights into the involvement of the central auditory system and neuroinflammation in tinnitus mechanisms.
On the other hand, the gut microbiota has played an essential role in regulating the neuroendocrine system via the gut-brain axis, maintaining immune homeostasis, and preventing chronic inflammation. A dysbiosis condition of the gut microbiota will disrupt the gut-brain axis and stimulate an inflammatory process, which may cause tinnitus. Hence, this literature review focuses on gut dysbiosis and its effect on neurotransmitter and immune homeostasis, particularly neuroinflammation, and its role in the development of tinnitus, which is expected to become a theoretical framework for further research on the pathomechanism or therapeutic approach for tinnitus.
Methods
This research is done using a qualitative study design with the literature study approach encompassing literature seeking, identifying, analyzing, and interpreting previous studies that discussed factors related to gut microbial dysbiosis, neurotransmitters, neuroinflammation, and its implication in tinnitus. Literature searching was done from October 2021 until January 2022 and the strategy used was based on the Preferred Reporting Items for Systematic Reviews and Meta-analyses Statement (PRISMA). PubMed was the major search engine to collect literature in the full-text original article published between January 2016 and December 2021. In addition, reports from other sources, such as manual searches through the list of found study references that have relevant topics, were also used in this study. Some keywords accounted for the searching process: Gut Microbiome OR Tinnitus AND (“Neurotransmitter” OR Serotonin OR Dopamine OR Tinnitus Signaling); Microbiota-Gut-Brain-Axis AND Neuroinflammation OR Proinflammatory Cytokines OR TNF-α OR IL-6.
The inclusion criteria are literatures (1) in English; (2) published between January 2016 until December 2021; (3) in the form of full text original article; (4) an experimental study, inclusive of in vivo, in vitro, and human studies; (5) discusses about gut microbiome, gut dysbiosis, gut-brainaxis, neurotransmitter, neuroinflammation, and tinnitus signaling. Literature discusses other than neurological symptoms or diseases was excluded.
Results
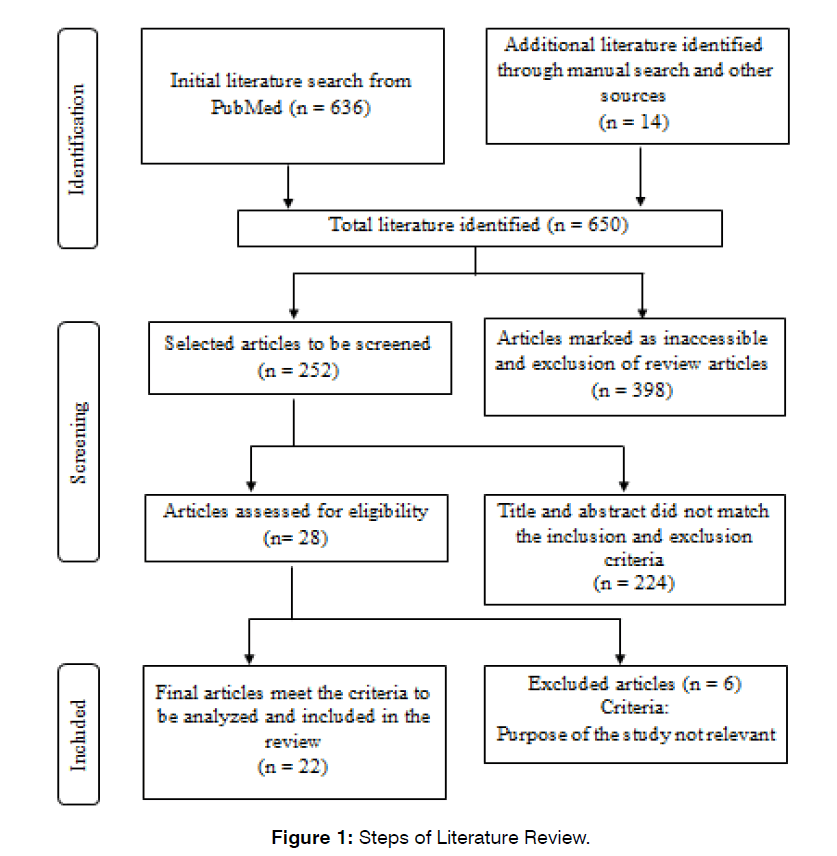
In this study, we found 650 articles from an initial search on PubMed and other sources, and the literature continued for further selection was 252 original articles. Subsequently, we selected 28 writings by title and abstract. After a complete reading of the text, some literature did not meet the inclusion criteria, resulting in 22 articles relevant to this study (Figure 1).
Figure 1: Brazilian Scale of Hearing and Language Development.
The main focus of this study is the pathophysiology of tinnitus concerning neurotransmitter activity and neuroinflammation associated with gut microbiota dysbiosis. However, some data need to be considered, such as the influence of gut microbiota and gut dysbiosis on neurotransmitters and neuroinflammatory mediators’ production, brain neurotransmitters associated with tinnitus, and neuroinflammatory mediators’ profile in conditions related to tinnitus.
The gut microbiome has attracted the attention of many researchers in recent years. The ability of gut microbiota to communicate with the host via the gut-brain axis by modulating the neurotransmitter production or activity may underlie the neural plasticity involved in generating tinnitus. Evidence showed that several neurotransmitters’ production increase following the presence of gut microbiota, as provided in Table 1 below.
| Authors | Sample | Intervention | Methods | Microbiota | Outcome | |
|---|---|---|---|---|---|---|
| Indicator | Results | |||||
| Luck et al. 2021 | Gut and brain tissues of B. dentium mono-associated mice and germ-free mice Mouse fecal |
None | Culture in ZMB1, Liquid Chromatography-Tandem Mass Spectrometry, IMG database, KEGG database | Bifidobacterium dentium ATCC 27678 | Gene involved in GABA, dopamine, epinephrine, tyrosine, norepinephrine producing pathway GABA |
B. dentium generate GABA from glutamate or succinate in the intestinal (but not in brain) by harboring the gene involved in GABA production. B. dentium modulate tyrosine in the gut and brain. B. dentium unable to synthesize either dopamine, epinephrine, or norepinephrine. |
| Duranti et al. 2020 | Human fecal Colon biopsy B. adolescentis supplemented Male wild-type Groningen rats (R. norvegicus) and control rats |
Supplementation of GMS in culture media Supplementation of B. adolescentis ATCC15703, PRL2019, HD17T2H, sucrose in rats for 5 days |
Culture on MRS media, HPLC, qPCR, qRT-PCR and ELISA | Bifidobacterium adolescentis | GABA, gene involved in GABA production | 79% of 82 B. adolescentis strains produce GABA from precursor monosodium glutamate (GMS). 23% of 82 B. adolescentis strains were classified as high GABA producers. Rats treated with GABA-producer B. adolescentis (PRL2019 and HD17T2H strain) and non-GABA-producer B. adolescentis ATCC15703 strain showed higher GABA concentrations compared to the control group. Expression of gadB and gadC of PRL2019 and HD17T2H were enhanced, showed that PRL2019 and HD17T2H can stimulate GABA production in rat model. |
| Strandwitz et al. 2019 | Adult healthy human fecal KBase database |
None | Co-culture on Molten FAAy, LC/MS In Silico using Bidirectional Best Hit (BBH) nucleotide BLAST |
KLE1738 Bacteroides ssp. Parabacteroides sp. E. coli Bifidobacterium |
GABA | KLE1738 requires GABA as carbon and energy source for growth. Bacteroides, Parabacteroides, Eubacterium, Bifidobacterium can produce GABA in co-culture assay. Bacteroides ssp. and Parabacteroides sp. produce GABA in normal pH range of the human large intestine. 97 organisms of the gut microbiota had the capability to produce GABA, >25% of these were Bacteroides and Parabacteroides. |
| Villageliu et al. 2018 | Animal fecal, cecal contents, rumen contents |
None | sSIM and UHPLC-ECD |
Enterococcus faecium Lactobacillus plantarum |
Tyrosine, Acetylcholine | E. faecium strain ML1085, ML1087, and ML1089 utilize tyrosine to produce tyramine, with ML1085 as the most rapid one in utilizing tyrosine. L. plantarum produce acetylcholine (average of 4.02μgmL-1) |
| Engevik et al. 2021 | Mouse small and large instestine, fecal. Human intestinal epithelial cell culture. Mouse and human enteroids culture |
Adult germ-free mice treated with sterile media, live Bifidobacterium dentium, heat-killed B dentium, or live B ovatus | Immunostaining, qPCR, LC-MS/MS | Bifidobacterium dentium Bifidobacterium ovatus |
5-HT, Htr2a (excitatory serotonin receptor) | B. dentium has a functional impact on enterochromaffin-produced serotonin B dentium mono-associated mice showed greter numbers of 5-HT-positive cells in the ileum and colon compared to the germ-free and heat-killed B dentium-treated mice. In the intestinal lumen of B. dentium mono-associated mice: Tph1 gene expression and 5-HT increased. Human intestinal enteroids (HIEs) microinjected by B. dentium CM showed higher levels of 5-HT. Htr2a expression was increased in the CA1 region of hippocampus in B. dentium mono-associated mice. No changes found in the concentrations of Tph1 and serotonin in B. ovatus mono-associated mice compared with the germ-free controls. |
| Mandic et al. 2019 | Intestinal cells and organoids of germ free male C3H/HeOuJ mice and C. ramosum mono-associated mice. | 1.Mono-association with C. ramosum DSM 1402 Feeding mice with low fat diet (LFD) or high fat diet (HFD) |
Immunofluorescence, ELISA, RT-PCR, western blot | Clostridium ramosum | 5-HT, ChA, gene involved in 5-HT metabolism | Colonic serotonin-producing (5-HT+) and chromogranin A-positive (ChA+) enteroendocrine cells C. ramosum-mice increased. Gene involved in 5-HT metabolism (Tph1 and Maoa) in C. ramosum-mice increased. eWAT also increased in 5-HT levels of C. ramosum-mice. |
Table 1: Neurotransmitter Modulation by the Gut Microbiota
GABA is one of the major inhibitory neurotransmitters in the brain that is reported to decrease in tinnitus. Table 1 describes several in vitro studies proving that gut microbiota can produce GABA, particularly Bifidobacterium, Bacteroides, Parabacteroides, and Eubacterium. have examined the genome of 83 Bifidobacteria species and found out that 30 out of 83 species contain succinate-semialdehyde dehydrogenase involves in the conversion of succinate to GABA, while 79 out of 83 species possess the genes to produce GABA from glutamate or glutamine. In vitro study by also showed a similar result: Bifidobacterium contains Gad genes involved in GABA production, and Bifidobacterium adolescentis could generate GABA from precursor GMS.
On the other hand, serotonin or 5-HT is a neurotransmitter involved in enhancing the firing rate in the auditory pathway [24]. Studies conducted and Mandic et al. (2019) showed that gut microbiota, namely Bifidobacterium dentium and Clostridium ramosum could regulate the production of 5-HT but not directly from de novo synthesis of 5-HT due to the lack of genes involved in 5-HT production. However, they increase the 5-HT positive enteroendocrine cells, Tph1 and Maoa genes expression to stimulate neurotransmitter production. Moreover, the B. dentium secreted products, including acetate, and C. ramosum cells lysates, can also stimulate enterochromaffin cells to produce 5-HT. Other microbiota, namely Enterococcus faecium, has shown the ability to utilize tyrosine to produce tyramine. In contrast, Lactobacillus Plantarum has been reported to produce acetylcholine in a pantothenic acid-supplied medium [21].
Furthermore, any disruption of the microbiota composition resulting in gut dysbiosis may also affect the neurotransmitter activity or production in the body. Table 2 shows the implications of the dysbiosis gut microbiota induced by several factors, such as a highfat diet, antibiotics, and other neurological conditions, on neurotransmitter activity (Figure 2).
| Authors | Subject | Intervention | Methods | Microbiota | Outcome | |
|---|---|---|---|---|---|---|
| Indicator | Results | |||||
| Guo et al. 2021 | Adult male KM mice | Administration of HSHF diet | UPLC-MS/MS, RNA sequencing |
phyla Acidobacteria, Verrucomicrobia, Tenericutes, Bacteroidetes, Proteobacteria, Deferribacteres, Cyanobacteria and Actinobacteria | Bacteria OTUs, amine oxidase, COMT, monoamine neurotransmitter | The HSHF diet induced dysbacteriosis in mice showed by decreased in the OTUs of bacteria of the phyla Verrucomicrobia, Acidobacteria, Tenericutes, and Firmicutes, while the OTUs of bacteria phyla Actinobacteria, Bacteroidetes, Deferribacteres, Proteobacteria, and Cyanobacteria were increased. HSHF diet induced dysbacteriosis in mice affect the level of Amine oxidase (MAOA and MAOB) and also COMT that play a role in oxidizes biogenic amines e.g., 5-HT, epinephrine, norepinephrine, and dopamine. HSHF diet induced changes in the level of some monoamine neurotransmitters in the brain. |
| Wu et al. 2020 | Male adult C57BL/6 mice exposed with CRS and control mice | Exposure with chronic restraint stress (CRS) | 16S rRNA gene sequence & Liquid and gas chromatography mass spectroscopy | genus Allobaculum and Akkermansia | Bacteria taxa genus Allobaculum and Akkermansia, norepinephrine, 5-HIAA, 5-HT | Gut microbiota composition and neurotransmitter composition in depressed and control mice were significantly different. Bacteria genus Allobaculum and Akkermansia was the least abundant taxa in depressed mice compared to control. Norepinephrine, 5-HIAA, and 5-HT levels were significantly decreased in depressed mice. 5-HT was positively correlated with two bacteria taxa: genus Allobaculum and genus Akkermansia. Norepinephrine and family Spirochaetaceae and genus revotellaceae_NK3B31_group were positively correlated. 5-HIAA were positively correlated with Alphaproteobacteria and negatively correlated with genus norank_f_Eggerthellaceae. |
| Gao et al. 2018 | Twelve male Duroc x Landrace x Large White weaned piglets | Ileal infusion of either saline or antibiotics | 16S rRNA MiSeq sequencing, hypothalamic transcriptome sequencing, qRT-PCR, and HPLC, ELISA |
Lactobacillus, Bifidobacterium, Euryarchaeota, Spirochaetes, Tenericutes, | Lactobacillus, Bifidobacterium, Euryarchaeota, Spirochaetes, Tenericutes, GABA, dopamine, 5-HT, BDNF, AAAs | Antibiotic infusion in the distal ileal altered the microbiota composition in the colon. Lactobacillus and Bifidobacterium species in the colon were increased following antibiotic infusion, while Euryarchaeota, Spirochaetes and Tenericutes’s relative abundance in the feces were significantly decreased. GABA, dopamine, 5-HT, and BDNF concentration in hypothalamus and blood were significantly decreased after antibiotic infusion. An alteration in the gut microbiota following antibiotic infusion correlated with decreased concentration of AAAs in circulation and hypothalamus which also accompanied by the decreased in 5-HT and dopamine concentrations in the hypothalamus. |
| Labban et al. 2020 | Fecal and brain tissues of twelve male Wistar albino rats (6 controls & 6 HFD fed rats) | Administration of HFD | ELISA, bacterial culture | Gram negative bacteria, Clostridium, and Bacteroides | Dopamine, glutamate, serotonin, bacterial composition | Dopamine and glutamate levels were significantly higher while serotonin levels were significantly lower in brain tissue of the rats treated with high fat diet (obese rats) compared to the lean (control) rats. Bacterial composition changes were noted following high fat diet intake. Gram negative bacteria, Clostridium, and Bacteroides were dominant in obese group compared to the control group which gram-positive bacteria was dominant. |
| Zhu et al. 2020 | Acutely relapsed schizophrenic (ARSCZ) and first-episode schizophrenic (FESCZ) patients & healthy controls Male C57BL/6 J mice transplanted with bacteria or saline |
Transplantation of S. vestibularis ATCC 49124 or S. thermophilus ST12, or saline (control) via oral gavage | Shotgun metagenomic sequencing, ELISA, LC-MS, UHPLC-MS | S. vestibularis | α diversity, β diversity, dopamine, GABA, 5-HT, tryptophan | Gut microbial α diversity at the genus level was greater and β diversity at the genus and microbial gene level was higher in schizophrenic patients. Akkermansia muciniphila, Bacteroides plebeius, Clostridium symbiosum, Eubacterium siraeum, Cronobacter sakazakii/turicensis, Veillonella parvula, S. vestibularis, Alkaliphilus oremlandii, Enterococcus faecium, Bifidobacterium longum, and Bifidobacterium adolescentis were significantly enriched in schizophrenia Dopamine levels in serum, intestinal contents, and colonic tissue were significantly lower in S. vestibularis-treated mice, and GABA levels in the intestinal contents were decreased shortly after transplantation. 5-HT levels were increased in the intestinal contents of S. vestibularis-treated mice throughout the behavioral test. Neurotransmitter levels in the brain were not affected by the S.vestibularis transplantation, but tryptophan levels decreased in prefrontal cortex of S. vestibularis-treated mice. |
Table 2: Study on Neurotransmitter Alteration in Dysbiosis Gut Microbiome
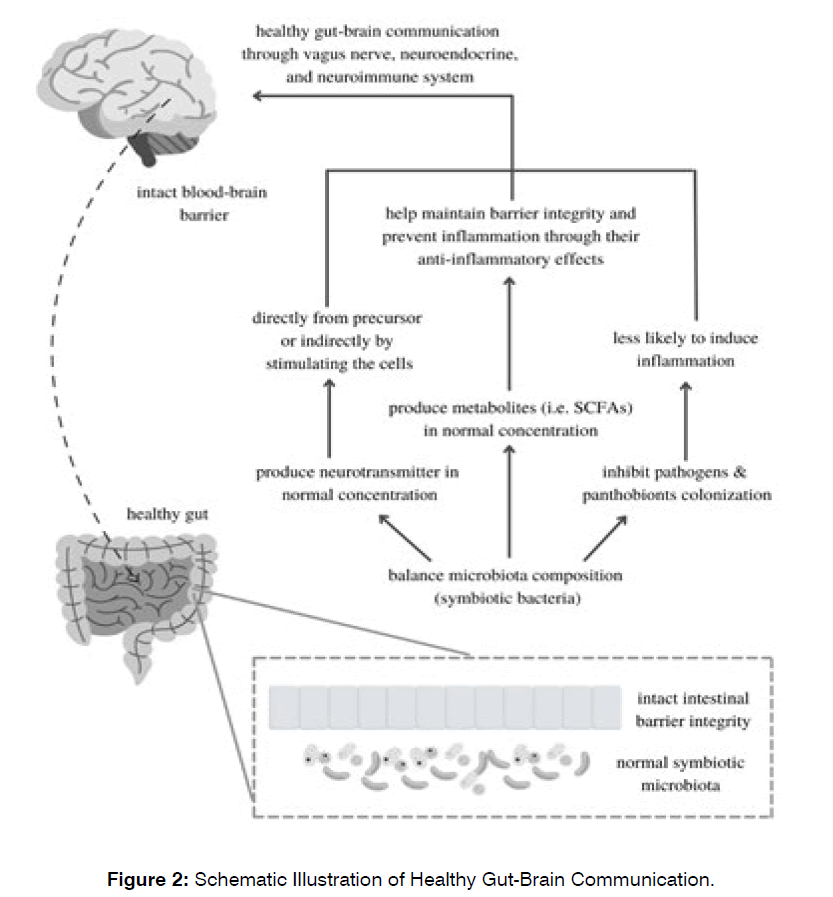
Figure 2: Brazilian Scale of Hearing and Language Development.
One of the main characteristics of gut dysbiosis is an increase or decrease in abundance, diversity, or operational taxonomic units (OTUs) of some bacteria taxa. Guo et al. identified an increased bacteria OTUs phyla Bacteroidetes, Proteobacteria, Deferribacteres, Cyanobacteria and Actinobacteria following an HSHF diet in mice, while Labban et al. (2020) found that HFD fed rats had a higher abundance of Gram-negative bacteria, Clostridium, and Bacteroides [17,25]. Moreover, another study conducted by Wu et al. (2020) reported a positive correlation between 5-HT levels and bacteria taxa genus Allobaculum and Akkermansia found to be least abundant in depressed mood mice. Different studies observed some changes in the gut microbiota diversity in schizophrenic patients. Gut microbiota in schizophrenic patients tends to be more varied compared to control, with a greater α and β diversity. Furthermore, they also observed some changes in neurotransmitter levels in mice transplanted with S. vestibularis, one of the bacteria found to increase in schizophrenic patients. Interestingly, the concentrations of dopamine and GABA decreased while 5-HT levels increased in the intestinal and serum of mice transplanted with S. vestibularis, but no changes in the brain. Besides, this study also showed a negative correlation between lower serum tryptophan levels and 38 bacterial enriched-schizophrenic patients, and higher levels of kynurenic acid (KYNA) positively correlated with ten bacterial enriched schizophrenics [26].
In addition, gut dysbiosis, also associated with an altered gut barrier, subsequently leads to the impairment of bloodbrain barrier permeability and neuroinflammation, which may contribute to the generation of several neurological diseases [27]. Table 3 describes some findings of the impact of gut dysbiosis on neuroinflammation, mainly through the activation of some proinflammatory mediators and pathways (Figure 3).
| Authors | Subject | Intervention/ Treatment |
Methods | Outcome | |
|---|---|---|---|---|---|
| Indicator | Result | ||||
| Wu et al. 2017 | Drosophila infected amyloid transgenic (Aβ42) | Enteric dysbiosis induced by oral infection with Ecc15 | Western blotting, immunostaining, qRT-PCR | TNF-α expression, JNK activity, AMPs | TNF-α was upregulated in the brain of the Drosophila infected amyloid transgenic (Aβ42) and further dysregulation occurred following enteric dysbiosis induced by oral infection with Ecc15. JNK activity increased in flies expressing Aβ42 with Ecc15 infection. Four different AMPs (Dpt, Drs, AttA, CecA1) were upregulated in the amyloid transgenic Drosophila brain after enteric infection. |
| Zhu et al. 2020 | Schizophrenia patients & healthy controls. Male C57BL/6J mice |
LPS stimulation | Shotgun metagenomic sequencing, qPCR gas chromatography–triple quadrupole mass spectrometry (GC-QQQ-MS) assay, immunoassay |
SCFAs, α-diversity of gut microbiota, immune signature | In Schizophrenia patients: total concentrations of SCFAs increased and positively correlated with the increase of α-diversity of gut microbiota. In Schizophrenia patients with immune-activated (IA): greater α-diversity included bacteria that produced SCFAs. TNF-α, IL-1β, IL-6 secretion and NF-κB activation (LPS-stimulated) enhanced with pre-incubation of SCFAs in the PBMCs of IA patients. In PBMCs IA patients: levels of mRNA of GPR41, GPR43, NF-κB, TNFα, T-bet, RORC, STAT3, IL-1B, IL-6, and IL-17a increased, and level of Foxp3 and IL-10 decreased. IL-6 and TNF-α in serum, IL-6 in PFC and hippocampus of adult offspring of MIA mice were upregulated after SCFA intake. |
| Labban et al., 2020 | Male Wistar albino rats | High Fat Diet (HFD) intake | ELISA, bacterial culture | Bacterial composition, Neuroinflammatory cytokines (IL-6 and Il-12). | Bacterial composition changes following HFD intake In HFD-rat (obese group): Gram negative bacteria, Clostridium, and Bacteroides were dominant In control group: Gram-positive bacteria was dominant. Cytokines IL-6 and IL-12 higher in HFD-rats. Those cytokines showed negative correlation with serotonin and positive correlation with dopamine and glutamate. |
| Lin et al. 2019 | Parkinson's diseases (PD) patients & healthy controls. | None | 16 rRNA gene amplicon and sequencing & multiplex immunoassay | Plasma pro-inflammation cytokine, Gut microbiota diversity and richness, | TNF-α and IFN-g plasma levels increased in PD. Gut microbiota in PD more diverse and richer, with relative abundance of Akkermansia, Parabacteroides, Verrucomicrobia, Butyricimonas, Enterococcus, Veillonella, Odoribacter, Mucispirillum, Bilophila, and Lactobacillus. Bacteroides abundance showed positive correlation with TNF-α plasma concentrations Verrucomicrobia abundance showed positive correlation IFN-g plasma concentrations. |
Table 3: Profile of Inflammatory Mediator in Dysbiosis Gut Microbiome
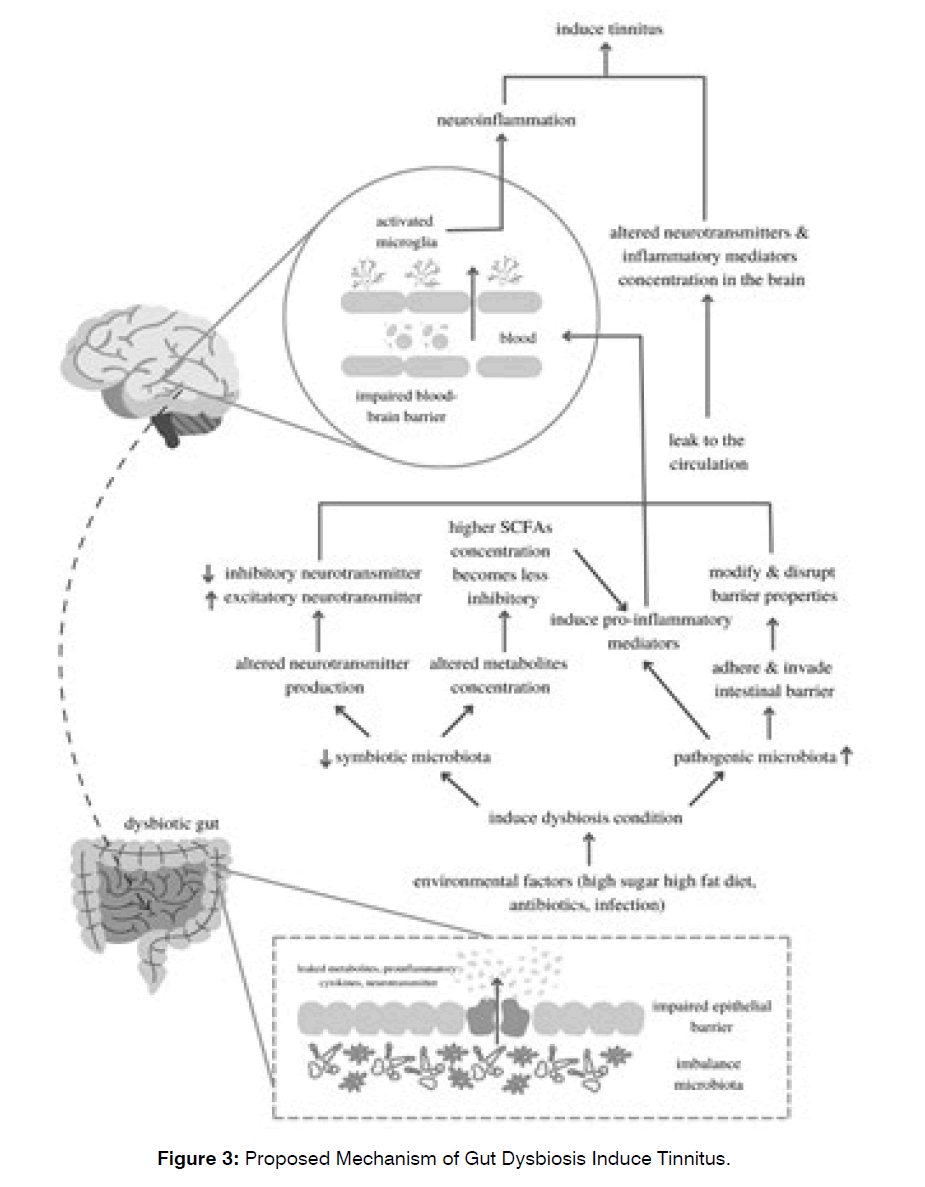
Figure 3: Brazilian Scale of Hearing and Language Development.
Numerous critical pro-inflammatory cytokines, namely TNF-α, IFN-γ, IL-6, and IL-12, were increased in gut dysbiosis induced by several factors, including bacterial infection, high fat diet, and medical conditions such as Alzheimer’s and Parkinson’s diseases [25,28,29]. Furthermore, analyzed a positive correlation between plasma concentrations of TNF-α and Bacteroides abundance and between IFN-γ and Verrucomicrobia in Parkinson patients suggesting that microbiota alteration contributes to the systemic inflammation involved in the disease progression [29].
On the other hand, different studies showed increased concentrations of SCFAs in immune-activated schizophrenic patients. SCFAs is a bacterial metabolite that has essential functions in maintaining the gut epithelial barrier and has an anti-inflammatory effect by increasing anti-inflammatory and decreasing the proinflammatory cytokines [30]. However, reported an increase in several proinflammatory cytokines in immune-activated schizophrenic patients and mice model of schizophrenia following SCFAs supplementation [31].
This result may be related to the findings that showed excessive concentration of SCFAs can be less inhibitory, thus can increase the production of pro-inflammatory cytokines, inducing an inflammatory response instead of their known anti-inflammatory effect [31-33].
While there is no firm evidence of gut dysbiosis involvement in tinnitus yet, several studies have described some changes in the neurotransmitters’ activity and neuroinflammatory mediators in the central auditory pathway associated with tinnitus; this may indicate the role of gut dysbiosis in the tinnitus mechanism. One of the neurotransmitters altered in tinnitus is dopamine. As shown in Table 4, the extracellular dopamine levels decreased in mice following salicylate-induced tinnitus [34]. Dopamine is a monoamine neurotransmitter that can exert heterogeneous effects in the auditory system. Dopamine can increase or decrease neural activity, although the most significant impact is reducing its responsiveness [35]. Another study found a decrease in Gabra1 mRNA, which encodes α1 subunits of GABAA receptors after auditory nerve damage. They also noted changes in excitatory/inhibitory indices in the auditory cortex and inferior colliculus of denervated mice that tend towards hyperexcitability [36]. Besides, Deng et al. (2020) have examined the occurrence of tinnitus was prevented by the infusion of M-8324, thus increasing the GABAergic inhibitory responses [37].
| Authors | Subject | Intervention/ Treatment |
Methods | Outcome | |
|---|---|---|---|---|---|
| Indicator | Result | ||||
| Tang & Trussell, 2017 | C57BL/6J wild-type mice or transgenic GlyT2-EGFP mice brain slices preparation | Bath application of 10 μM 5-HT | Electrophysiology and paired recordings | Spontaneous firing, EPSCs, PPR | The spontaneous firing of fusiform cells was enhanced after 5-HT application. Application of 5-HT in mice reduced the EPSCs of the Auditory Nerve Fiber (ANF) and increase its PPR, yet did not affect the PF EPSCs and PPR. 5-HT depolarized the membrane potential and increased the spontaneous spike activity of vertical cells, but did not affect the resting membrane and spike activity in cartwheel cells. The inhibitory synapses were not directly affected by 5-HT. |
| Deng et al. 2020 | C57BL/6J mice cell and brain slices and GAD67-GFP knock-in mice | Implantation of osmotic pump & exposure of pure tone 8 kHz noise | In vitro and in vivo electrophysiology, gap detection, immunofluorescence | Spontaneous and sound-evoked spiking, gap detection | An NMDAR-PAMs, M-8324, selectively increases NMDAR responses on inhibitory neurons. M-8324 increased spontaneous and sound-evoked spiking in GABAergic inhibitory neurons and decreased the responses of excitatory neurons, but the decreased was greater in spontaneous than sound-evoked spiking. M-8324 was found to prevents tinnitus by significantly suppressing the deficit in gap detection in noise exposure model compared to the Veh group. |
| Wang et al. 2021 | Healthy adult male Sprague-Dawley rats | Intraperitoneal administration of 350 mg/kg sodium salicylate | GPIAS, High Performance Liquid Chromatography and electrochemical detection | Extracellular dopamine | GPIAS was significantly decreased at 12 kHz and 16kHz in rats 2 hours after SS treatment. Extracellular dopamine levels were significantly decrease in caudate putamen rats treated with sodium salicylate compared to the saline (control) group in which the levels of extracellular dopamine remained stable. |
| Balaram et al. 2019 | CBA/CaJ male mice Transgenic mice C57BL6 |
Administration of ouabain solution (for unilateral cochlear denervation) and distilled water (for control) Short term unilateral deafening with sterile distilled water |
Fluorescent in situ hybridization and quantitative image analysis | Gria2 mRNA, Gabra1 mRNA, excitatory/inhibitory indices | After 30 days of contralateral auditory denervation, the levels of Gria2 mRNA which encodes GluA2 subunits of AMPA was increased and the levels of Gabra1 mRNA transcripts which encodes α1 subunits of GABAA receptors was decreased in both inferior colliculus and auditory cortex. Gria2 elevations and Gabra1 reductions was more pronounced in auditory cortex than inferior colliculus. Excitatory/Inhibitory indices were more positive (tended towards hyperexcitability) in both auditory cortex and inferior colliculus of denervated mice compared to the control group. The reduction of Gabra1 and Gria2 transcription levels were equivalent in shorter period of cochlear denervation (5 days), there was no clear change in Excitatory/Inhibitory indices in mice induced short-term unilateral deafness compared to the control. |
Table 4: Profile of Neurotransmitter Activity in Central Auditory Pathway
In addition, several studies have been conducted to assess the changes in some neuroinflammatory mediators, particularly following noise exposure and salicylate injection, as provided in Table 5. Furthermore, activation of neuroglia and microglia characterized by the changes in shape from ramified into an activated non-ramified or amoeboid form were also noted in noise-induced and salicylate-induced tinnitus, proving the contribution of neuroinflammation in tinnitus [15,38].
| Authors | Subject | Intervention/ Treated |
Methods | Outcome | |
|---|---|---|---|---|---|
| Indicator | Result | ||||
| Wang et al. 2019 | Wild type mice (C57BL/6J) and TNF-α knockout mice |
Noise exposure TNF-α infusion PLX3397 (inhibitor of CSF1R) injection. 3,6'-dithiothalidomide (dTT) TNF-α inhibitor). |
RT-qPCR Gap detection test |
TNF-α expression., Microglia shape, Gap detection performance. | TNF-α mRNA level increased in wild type mice following noise exposure. Microglia shape were non-ramified or amoeboid on 5-day post noise exposure in wild-type mice, but remained ramified in knockout mice. Gap detection performance altered in wild-type mice but not in TNF-α knockout mice, but altered gap detection performance in wild-type mice and TNF-α knockout mice was found after TNF-α infusion. PLX3397 intraperitoneal injection for 21 days prevented an increase expression of TNF-α after noise exposure and also results in improved gap detection performance. Administration of 3,6'-dithiothalidomide (dTT) prevented an increase in TNF-α expression and microglia morphological changes after noise exposure. Also prevented increasing of other proinflammatory cytokines (IL-1β, IL-18, and NLRP3) 10 days after noise exposure. |
| Chen et al. 2017 | Mice Treated Untreated Rehabilitated |
Salicylate injection | RT-qPCR, western blot analysis | TNF-α gene, NR2A mRNA and protein, IL-6 mRNA and protein | TNF-α gene expression upregulated in chronic salicylate injection NR2A mRNA and protein expression upregulated in mice treated with salicylate for 7 continuous days which developed tinnitus-like behaviour compared to the control group, acute treatment group, and rehabilitation group. IFN-g gene expression downregulated in mice treated with salicylate chronically for 7 days. IL-6 mRNA and protein expression not altered in any group. |
| Deng et al. 2020 | Male C57BL/6 mice | Noise exposure TNF-α infusion |
Gap detection test, PPI test, immunofluorescence staining | Gap detection performance | TNF-α infusion and noise exposure caused impairment in both gap detection performances and PPI, but TNF-α infusion or noise exposure alone did not impaired both. TNF-α infusion and noise exposure caused changes in microglial morphology (deramification), but noise exposure or TNF-α infusion alone did not cause microglial deramification. Only mice that had received TNF-α infusion and noise exposure showed a reduction in PV+ neuron density. |
| Xia et al. 2020 | Male Sprague-Dawley rats | Salicylate injection | Western blotting, qPCR, immunohistochemical staining | GFAP and Iba1 gene, Astrocyte EP/C, Microglia EP/, IL1β protein | GFAP expression increased both in A1 cortex and MGB at 4 hours after acute and chronic salicylate treatment. Number of astrocyte EP/C in A1 cortex increased following chronic salicylate treatment, but not in the MGB. Iba1 expression increased both in A1 cortex and MGB after chronic salicylate treatment, but no obvious changes in microglia morphology both in A1 cortex and MGB Number of microglia EP/C increased in MGB after chronic salicylate treatment. IL-1β protein level increased in A1 cortex after acute and chronic salicylate treatment, but the level of IL-1β almost constant in MGB of mice treated with chronic salicylate. |
Table 5: Report Study of Inflammation Mediator Profile Following Noise Exposure and Salicylate Injection
Wang et al. found that TNF-α concentrations increased after noise exposure in mice. They also observed alteration in gap detection performance in wild-type, and TNF-α infused mice but not in TNF-α knockout mice. Also demonstrated that mice exposed to noise exposure and TNF- infusion experienced impaired gap detection performance and PPI. However, changes did not occur with exposure to TNF-α or noise alone. In addition, they also noted changes in microglial morphology to non-ramified or activated amoeboid forms after noise exposure and TNF-α infusion, whereas TNF-α knockout mice showed no changes [15,39].
In another study, observed an increase in TNF-α gene expression and NR2A mRNA levels after chronic salicylate injection for seven days continuously in mice, in contrast to IFN-γ gene expression tended to decrease [40]. Meanwhile, explored neuroglial activation in salicylateinduced tinnitus. They found an increased expression of GFAP and Iba1, specific markers for astrocyte and microglia activation, respectively, in cortex A1 and MGB after salicylate treatment. In addition, there was an increase in IL-1β protein levels in the A1 cortex of mice after acute and chronic salicylate injection [38-42].
DISCUSSION
In general, based on the putative mechanisms that have been studied, tinnitus can be divided into cochleartype and central-type. Cochlear-type tinnitus suggests aberrant activity in the peripheral auditory system, which could happen at the pre-cochlear or cochlear nerve level. Spontaneous Otoacoustic Emissions (SOAEs), hair cells damaged induced by noise exposure or ototoxic agents are some factors that contribute to this cochlear mechanism, altering the endocochlear potential to propagate through the auditory pathway to the cortex, generating tinnitus [43]. Meanwhile, the central-type tinnitus hypothesized that tinnitus generation is primarily influenced by the aberrant neural activity in the central auditory system and not by pre-existing cochlear damage [43]. These proposed mechanisms were strengthened by some studies showing that sectioning the auditory nerve bilaterally did not necessarily terminate the noise perception in tinnitus patients. Since then, many studies have been conducted to better understand the central mechanism of tinnitus.
Of this central-type tinnitus, two significant factors have been thought to contribute to the development of tinnitus, specifically the increased central gain about the maladaptive neural plasticity and neuroinflammation. A reduction or loss of auditory input may result in the decrease of inhibitory neurotransmitters. Additionally, the excitatory neurotransmitter increases, causing a shift in excitatoryinhibitory balances [14,44]. Concurrently, neuroinflammation is defined by increased proinflammatory cytokines and microglial activation.
The Microbiota-Gut-Brain Axis and Neurotransmitter Production: The human gut microbiota refers to the community of trillions of commensal and symbiotic microorganisms living in our intestines. Gram-positive bacteria usually dominate the normal gut, and the most dominant bacterial phyla in the mammalian gut are Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria [25,45,46]. Maintaining this gut microbiota balance is essential to the whole body’s health. Numerous studies have shown that gut microbial community alterations may contribute to several diseases pathogenesis; among these are inflammatory bowel disease [47,48], obesity [49], multiple sclerosis [50], and neurodegenerative or neuropsychiatric disorders such as Parkinson’s [29], Alzheimer’s, [28] schizophrenia, 26 autism spectrum disorders [51], and depression [16].
Bidirectional communication exists between gut microbiota and CNS via the gut-brain axis. The bottomup communication is thought to involve mainly the metabolic, immune, endocrine, and neural systems via the autonomic vagus nerve. [32,45,52]. Bacterial metabolites, i.e. short-chain fatty acids (SCFAs), tryptophan metabolites, and secondary bile acids (2BAs), are mediators for this communication. These metabolites propagate signals mainly by interacting with Enterochromaffin Cells (ECCs), Enteroendocrine Cells (EECs), and the mucosal immune system. However, some can cross the intestinal barrier and enter systemic circulation or even cross the bloodbrain border [52,53].
Moreover, recent studies have shown that gut microbiota also plays a role in producing neuroactive molecules, either by directly producing them from the precursor or indirectly by stimulating the cells to produce neurotransmitters, thereby contributing to gut-brain signaling. Bifidobacterium, Bacteroides, Parabacteroides, and Eubacterium showed the ability to produce neurotransmitter GABA from the precursor glutamate or succinate. Besides, Lactobacillus have been shown to produce acetylcholine and tyramine [18-21] Additionally, Bifidobacterium and Clostridium can stimulate the 5-HT production by enterochromaffin cells, and it is estimated that around 90% of the serotonin needed for CNS function is produced by gut microbiota [22,23,46].
Gut Microbiota Dysbiosis and Neuroinflammation: One of the most important pathways for gut-brain communication is the neuroimmune system. There is increasing evidence that gut microbiota can modulate brain immune function through the production of inflammatory cytokines, reactive oxygen species, and microglia maturation and activation [54]. A study on germfree (GF) mice and specific-pathogen free (SPF) mice showed that the microglia mRNA genes profiles and the CNS histology in GF and SPF mice were markedly different. GF mice demonstrated a lower level of genes needed for cell activation and type I IFN receptor signaling. Furthermore, microglia in GF mice failed to evolve to their activated shape when induced by LPS injection, and the innate immune response was also found to be impaired in GF mice compared to SPF mice [55].
Another study showed a correlation between the abundance of bacteria genus Bacteroides and Verrucomicrobia. They increased plasma levels of TNF-α and IFN-γ in humans with Parkinson’s disease (PD) compared to the healthy control [29]. A Parkinson’s disease mice model also showed the gut microbiota involvement in modulating inflammatory cytokines, particularly IL-6 and microglia activation. Mice given PD-derived microbial donors developed an increased motor dysfunction and microglial activation compared to GF mice. Furthermore, bacterial metabolite SCFAs also showed the same results [56]. Hence, these results demonstrated the importance of gut microbiota in neuroinflammation and neurodegenerative disease.
In addition, numerous studies have provided evidence of the effect of intestinal microbiota dysbiosis on the integrity of the host epithelial barrier. For example, environmental factors such as infections, high-fat diet and antibiotics can cause dysbiosis characterized by reduced symbiotic microbiota and loss of essential metabolites; these can induce a pro-inflammatory response and intestinal leakage due to impaired intestinal barrier integrity [57,58]. Another study showed that the BBB permeability of mice lacking a normal gut microbiota was increased. This may be caused by the decreased expression of occludin and claudin-5, which is the transmembrane tight junction protein. Moreover, recolonization with more complex microbiota and SCFAs producing bacteria improved the BBB integrity [54,59]. Taken together, this gut dysbiosis, intestinal epithelial barrier disruption, neuroinflammation, and loss of blood-brain barrier integrity may contribute to the pathogenesis of several neurological diseases.
The Potential Role of Gut Dysbiosis in Inducing Tinnitus:
The implications of gut dysbiosis on neurotransmitters production and neuroinflammation described above may also indicate the role of gut microbiota dysbiosis in inducing tinnitus. Environmental factors e.g. diet, infections and antibiotics may alter the beneficial microbiota needed for neurotransmitter production, causing the shift in neurotransmitter balance [25,32]. It has been previously described that the presence of tinnitus is related to the decrease in inhibitory neurotransmitters such as GABA and an increase in excitatory neurotransmitters44. Besides, the increased GABAergic inhibitory neurons and decreased excitatory responses have been successfully reported to prevent tinnitus, proving the role of neurotransmitter modulation in tinnitus [37].
In addition, any factors triggering the gut dysbiosis may also induce epithelial barrier dysfunction and microbial adhesion, which further elicits proinflammatory responses [57]. This increased proinflammatory cytokines and activation of microglia, the brain defense mechanism, together with the presence of other risk factors such as noise exposure and excess salicylate administration, then causing the behavioral evidence of tinnitus [15,39,40].
Conclusion
In conclusion, neurotransmitter production and neuroinflammatory modulatory responses are likely to be critical factors in the development of tinnitus. Meanwhile, the gut microbiota’s ability to build a complex cross-talk with their hosts to create the gut-brain axis is crucial for regulating the neuroendocrine system and maintaining immune homeostasis. However, microbial composition changes or dysbiosis can interfere with neurotransmitter production, causing hyperexcitability; and modulating neuroinflammatory responses through activation of microglia and several proinflammatory cytokines. Therefore, gut dysbiosis is likely to occur in tinnitus, inducing sound perception by altering those factors. Disruption of the gut barrier and BBB integrity may also contribute to this process, thus facilitating some bacterial metabolites and neuroactive molecules to reach the brain, altering its function and causing tinnitus.
References
- Atik A. Pathophysiology and treatment of tinnitus: an elusive disease. Indian J Otolaryngol Head & Neck Surg. 2014;66(1):1-5.
- Chung JH, Lee SH. The pathophysiologic mechanism of tinnitus. Hanyang Med Rev. 2016;36(2):81-5.
- Rosing SN, Schmidt JH, Wedderkopp N, Baguley DM. Prevalence of tinnitus and hyperacusis in children and adolescents: A systematic review. BMJ open. 2016;6(6):e010596.
- Bartnik G, Stepien A, Raj-Koziak D, Fabijanska A, Niedzialek I, Skarzynski H. Troublesome tinnitus in children: epidemiology, audiological profile, and preliminary results of treatment. Inte J pediatr. 2012.
- Bhatt JM, Lin HW, Bhattacharyya N. Prevalence, severity, exposures, and treatment patterns of tinnitus in the United States. JAMA Otolaryngol–Head & Neck Surg. 2016;142(10):959-65.
- Ziai K, Moshtaghi O, Mahboubi H, Djalilian HR. Tinnitus patients suffering from anxiety and depression: a review. The Int Tinnitus J. 2017;21(1):68-73.
- Yew KS. Diagnostic approach to patients with tinnitus. Am Family Phys. 2014;89(2):106-13.
- Makar SK. Etiology and Pathophysiology of Tinnitus-A Systematic Review. Int Tinnitus J. 2021;25(5).
- Kim HJ, Lee HJ, An SY, Sim S, Park B, Kim SW, et al. Analysis of the prevalence and associated risk factors of tinnitus in adults. PloS One. 2015;10(5):e0127578.
- Shargorodsky J, Curhan GC, Farwell WR. Prevalence and characteristics of tinnitus among US adults. Am J Med [Internet]. 2010;123(8):711–8.
- Henry JA, Roberts LE, Caspary DM, Theodoroff SM, Salvi RJ. Underlying mechanisms of tinnitus: review and clinical implications. J Am Academy of Audiol. 2014;25(01):005-22.
- Caspary DM, Llano DA. Auditory thalamic circuits and GABAA receptor function: putative mechanisms in tinnitus pathology. Hearing Res. 2017;349:197-207.
- Kalappa BI, Brozoski TJ, Turner JG, Caspary DM. Single unit hyperactivity and bursting in the auditory thalamus of awake rats directly correlates with behavioural evidence of tinnitus. J physiol. 2014;592(22):5065-78.
- Sedley W, Parikh J, Edden RA, Tait V, Blamire A, Griffiths TD. Human auditory cortex neurochemistry reflects the presence and severity of tinnitus. J Neurosci. 2015;35(44):14822-8.
- Wang W, Zhang LS, Zinsmaier AK, Patterson G, Leptich EJ, Shoemaker SL, et al. Neuroinflammation mediates noise-induced synaptic imbalance and tinnitus in rodent models. PLoS Biol. 2019;17(6):e3000307.
- Wu M, Tian T, Mao Q, Zou T, Zhou CJ, Xie J, et al. Associations between disordered gut microbiota and changes of neurotransmitters and short-chain fatty acids in depressed mice. Translational psychiatr. 2020;10(1):1-0.
- Guo Y, Zhu X, Zeng M, Qi L, Tang X, Wang D, et al. A diet high in sugar and fat influences neurotransmitter metabolism and then affects brain function by altering the gut microbiota. Translational Psychiatr. 2021;11(1):1-27.
- Luck B, Horvath TD, Engevik KA, Ruan W, Haidacher SJ, Hoch KM, et al. Neurotransmitter Profiles Are Altered in the Gut and Brain of Mice Mono-Associated with Bifidobacterium Dentium. Biomolecules. 2021;11(8):1091.
- Duranti S, Ruiz L, Lugli GA, Tames H, Milani C, Mancabelli L, et al. Bifidobacterium adolescentis as a key member of the human gut microbiota in the production of GABA. Scientific Reports. 2020;10(1):1-3.
- Strandwitz P, Kim KH, Terekhova D, Liu JK, Sharma A, Levering J, et al. GABA-modulating bacteria of the human gut microbiota. Nature Microbiol. 2019;4(3):396-403.
- Villageliú DN, Rasmussen S, Lyte M. A microbial endocrinology-based simulated small intestinal medium for the evaluation of neurochemical production by gut microbiota. FEMS Microbiol Ecol. 2018;94(7):fiy096.
- Engevik MA, Luck B, Visuthranukul C, Ihekweazu FD, Engevik AC, Shi Z, et al. Human-derived Bifidobacterium dentium modulates the mammalian serotonergic system and gut–brain axis. Cellular and Molecular Gastroenterol and Hepatol. 2021;11(1):221-48.
- Mandic AD, Woting A, Jaenicke T, Sander A, Sabrowski W, Rolle-Kampcyk U, et al. Clostridium ramosum regulates enterochromaffin cell development and serotonin release. Scientific reports. 2019;9(1):1-5.
- Tang ZQ, Trussell LO. Serotonergic modulation of sensory representation in a central multisensory circuit is pathway specific. Cell reports. 2017;20(8):1844-54.
- Labban RS, Alfawaz H, Almnaizel AT, Hassan WM, Bhat RS, Moubayed NM, et al. High-fat diet-induced obesity and impairment of brain neurotransmitter pool. Translational Neurosci. 2020;11(1):147-60.
- Zhu F, Ju Y, Wang W, Wang Q, Guo R, Ma Q, et al. Metagenome-wide association of gut microbiome features for schizophrenia. Nature Communications. 2020;11(1):1-0.
- Megur A, Baltriukiene D, Bukelskiene V, Burokas A. The Microbiota–Gut–Brain Axis and Alzheimer’s Disease: Neuroinflammation Is to Blame?. Nutrients. 2020;13(1):37.
- Wu S, Cao Z, Chang K, Juang J. Intestinal microbial dysbiosis aggravates the progression of Alzheimer’s disease in Drosophila. Nat Commun [Internet]. 2017;8(24):1–8.
- Lin CH, Chen CC, Chiang HL, Liou JM, Chang CM, Lu TP, et al. Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson’s disease. J Neuroinflammation. 2019;16(1):1-9.
- Martin-Gallausiaux C, Marinelli L, Blottière HM, Larraufie P, Lapaque N. SCFA: mechanisms and functional importance in the gut. Proceedings of the Nutrition Society. 2021;80(1):37-49.
- Zhu F, Wang W, Ma Q, Yang Z, Fan Y, Ju Y, et al. Role of short-chain fatty acids in the gut-brain axis in schizophrenia: contribution to immune activation and pathophysiology in humans and mice. Biorxiv. 2020.
- Gao K, Pi Y, Mu CL, Peng Y, Huang Z, Zhu WY. Antibiotics-induced modulation of large intestinal microbiota altered aromatic amino acid profile and expression of neurotransmitters in the hypothalamus of piglets. J Neurochemistry. 2018;146(3):219-34.
- Mirmonsef P, Zariffard MR, Gilbert D, Makinde H, Landay AL, Spear GT. Short-chain fatty acids induce pro-inflammatory cytokine production alone and in combination with toll-like receptor ligands. American J Reprod Immunol. 2012;67(5):391-400.
- Wang ML, Song Y, Liu JX, Du YL, Xiong S, Fan X, et al. Role of the caudate-putamen nucleus in sensory gating in induced tinnitus in rats. Neural Regeneration Res. 2021;16(11):2250.
- Hoyt JM, Perkel DJ, Portfors CV. Dopamine acts via D2-like receptors to modulate auditory responses in the inferior colliculus. Eneuro. 2019;6(5).
- Balaram P, Hackett TA, Polley DB. Synergistic transcriptional changes in AMPA and GABAA receptor genes support compensatory plasticity following unilateral hearing loss. Neurosci. 2019;407:108-19.
- Deng D, Masri S, Yao L, Ma X, Cao X, Yang S, et al. Increasing endogenous activity of NMDARs on GABAergic neurons increases inhibition, alters sensory processing and prevents noise-induced tinnitus. Scientific Reports. 2020;10(1):1-3.
- Xia C, Yin M, Wu C, Ji Y, Zhou Y. Neuroglial activation in the auditory cortex and medial geniculate body of salicylate-induced tinnitus rats. Am J Translational Res. 2020;12(10):6043.
- Deng D, Wang W, Bao S. Diffusible tumor necrosis factor-alpha (TNF-a) promotes noise-induced parvalbumin-positive (PV+) neuron loss and auditory processing impairments. Frontiers in Neurosci. 2020:1065.
- Chen XH, Zheng LL. Expression of pro-inflammatory cytokines in the auditory cortex of rats with salicylate-induced tinnitus. Molecular Med Reports. 2017;16(4):5643-8.
- Wang ML, Song Y, Liu JX, Du YL, Xiong S, Fan X, et al. Role of the caudate-putamen nucleus in sensory gating in induced tinnitus in rats. Neural Regeneration Res. 2021;16(11):2250.
- Balaram P, Hackett TA, Polley DB. Synergistic transcriptional changes in AMPA and GABAA receptor genes support compensatory plasticity following unilateral hearing loss. Neurosci. 2019;407:108-19.
- Noreña AJ. Revisiting the cochlear and central mechanisms of tinnitus and therapeutic approaches. Audiol and Neurotol. 2015;20(Suppl. 1):53-9.
- Shore SE, Roberts LE, Langguth B. Maladaptive plasticity in tinnitus—triggers, mechanisms and treatment. Nature Rev Neurol. 2016 ;12(3):150-60.
- Cryan JF, O'Riordan KJ, Cowan CS, Sandhu KV, Bastiaanssen TF, Boehme M, et al. The microbiota-gut-brain axis. Physiol Rev. 2019.
- Rutsch A, Kantsjö JB, Ronchi F. The gut-brain axis: how microbiota and host inflammasome influence brain physiology and pathology. Frontiers in Immunol. 2020:3237.
- Halfvarson J, Brislawn CJ, Lamendella R, Vázquez-Baeza Y, Walters WA, Bramer LM, et al. Dynamics of the human gut microbiome in inflammatory bowel disease. Nature Microbiol. 2017;2(5):1-7.
- Ni J, Wu GD, Albenberg L, Tomov VT. Gut microbiota and IBD: causation or correlation?. Nature Rev Gastroenterol & Hepatol. 2017;14(10):573-84.
- Amabebe E, Robert FO, Agbalalah T, Orubu ES. Microbial dysbiosis-induced obesity: role of gut microbiota in homoeostasis of energy metabolism. British J Nutr. 2020;123(10):1127-37.
- Saresella M, Marventano I, Barone M, La Rosa F, Piancone F, Mendozzi L, et al. Alterations in circulating fatty acid are associated with gut microbiota dysbiosis and inflammation in multiple sclerosis. Frontiers in Immunol. 2020:1390.
- Liu Z, Mao X, Dan Z, Pei Y, Xu R, Guo M, et al. Gene variations in Autism Spectrum Disorder are associated with alternation of gut microbiota, metabolites and cytokines. Gut Microbes. 2021;13(1):1854967.
- Martin CR, Osadchiy V, Kalani A, Mayer EA. The brain-gut-microbiome axis. Cellular and Molecular Gastroenterol and Hepatol. 2018;6(2):133-48.
- Osadchiy V, Martin CR, Mayer EA. The gut–brain axis and the microbiome: mechanisms and clinical implications. Clinical Gastroenterol and Hepatol. 2019;17(2):322-32.
- Schächtle MA, Rosshart SP. The microbiota-gut-brain axis in health and disease and its implications for translational research. Frontiers in Cellular Neurosci. 2021:256.
- Erny D, de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nature Neurosci. 2015;18(7):965-77.
- Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell. 2016;167(6):1469-80.
- Kinashi Y, Hase K. Partners in leaky gut syndrome: intestinal dysbiosis and autoimmunity. Frontiers in Immunol. 2021;12.
- Ochoa-Repáraz J, Kasper LH. The second brain: is the gut microbiota a link between obesity and central nervous system disorders?. Current Obesity Reports. 2016;5(1):51-64.
- Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Science Translational Med. 2014;6(263):158.
1Department of Biomedical Science, Division of Microbiology, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia
2Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia
3Department of Otorhinolaryngology-Head and Neck Surgery, Faculty of Medicine, Universitas Padjadjaran/Hasan Sadikin General Hospital, Bandung, Indonesia
4Department of Biomedical Science, Division of Physiology, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia
5Department of Otorhinolaryngology-Head and Neck, Santosa Hospital Bandung Central, Bandung, Indonesia
Send correspondence to:
Imam Megantara
Department of Biomedical Science, Division of Microbiology, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia, Eyckman no. 38, Bandung, West Java, Indonesia 40161, Email: imam.megantara@unpad.ac.id
Phone: +62 816 612 839.
Paper submitted on Feb 17, 2022; and Accepted on March 24, 2022
Citation: Imam Megantara, Gisela Liani Wikargana, Yussy Afriani Dewi, Agung Dinasti Permana, Nova Sylviana. The Role of Gut Dysbiosis in the Pathophysiology of Tinnitus: A Literature Review. Int Tinnitus J. 2022;26(1):27-41.